Protective agent for CAR-T cell cryopreservation and cryopreservation method
A cryopreservation method and protective agent technology, applied in the field of immune cells, can solve problems such as poor safety, inability to meet the needs of use, limited autologous plasma materials, etc., to maintain stability, prolong cryopreservation time, and reduce cell damage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
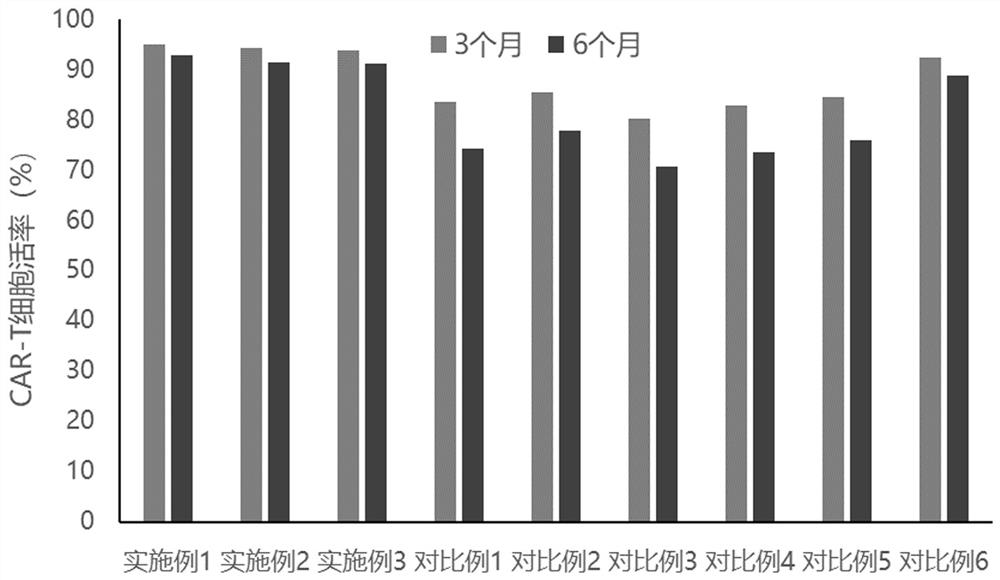
[0023] A protective agent for cryopreservation of CAR-T cells, consisting of RPMI1640 medium and the following components added to the medium: Paulownin, inositol nicotinate, wax gourd seed extract, hydroxytyrosol, β-glucan , insulin, glutathione; the final concentration of each component in the medium is: Paulownin 3ng / mL, inositol nicotinate 5μg / mL, wax gourd seed extract 2μg / mL, hydroxytyrosol 14ng / mL, β - Dextran 12 μg / mL, Insulin 7 μg / mL, Glutathione 8 μg / mL.
[0024] A cryopreservation method for CAR-T cells, mixing CAR-T cells and a protective agent to obtain a cell suspension, the density of CAR-T cells in the protective agent is 7×10 6 pcs / mL, divided into sterile cryopreservation tubes, 1.5mL per tube, the cryopreservation tubes were cooled to -80°C by 3°C / min program, and then transferred to liquid nitrogen for cryopreservation.
Embodiment 2
[0026] A protective agent for cryopreservation of CAR-T cells, consisting of RPMI1640 medium and the following components added to the medium: Paulownin, inositol nicotinate, wax gourd seed extract, hydroxytyrosol, β-glucan , insulin, glutathione; the final concentration of each component in the culture medium is: Paulownin 1ng / mL, inositol nicotinate 2μg / mL, wax gourd seed extract 1μg / mL, hydroxytyrosol 12ng / mL, β - Dextran 10 μg / mL, Insulin 4 μg / mL, Glutathione 5 μg / mL.
[0027] A method for cryopreserving CAR-T cells, mixing CAR-T cells and a protective agent to obtain a cell suspension, and the density of CAR-T cells in the protective agent is 5×10 6 pcs / mL, divided into sterile cryopreservation tubes, 1.5mL per tube, the cryopreservation tubes were cooled to -80°C by 3°C / min program, and then transferred to liquid nitrogen for cryopreservation.
Embodiment 3
[0029] A protective agent for cryopreservation of CAR-T cells, consisting of RPMI1640 medium and the following components added to the medium: Paulownin, inositol nicotinate, wax gourd seed extract, hydroxytyrosol, β-glucan , insulin, glutathione; the final concentration of each component in the medium is: Paulownin 5ng / mL, inositol nicotinate 8μg / mL, wax gourd seed extract 3μg / mL, hydroxytyrosol 16ng / mL, β - Dextran 14 μg / mL, Insulin 9 μg / mL, Glutathione 10 μg / mL.
[0030] A cryopreservation method for CAR-T cells, mixing CAR-T cells and a protective agent to obtain a cell suspension, the density of CAR-T cells in the protective agent is 9×10 6 pcs / mL, divided into sterile cryopreservation tubes, 1.5mL per tube, the cryopreservation tubes were cooled to -80°C by 3°C / min program, and then transferred to liquid nitrogen for cryopreservation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com