Therapeutic combinations comprising Anti-cd123 immunoconjugates
An immunoconjugate, BCL-2 technology, applied in the field of treatment of hematological malignancies, can solve the problems of inability to treat hematological malignancies and poor long-term survival of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0320] In vitro study of the combination of IMGN632 and venetoclax in AML cell lines
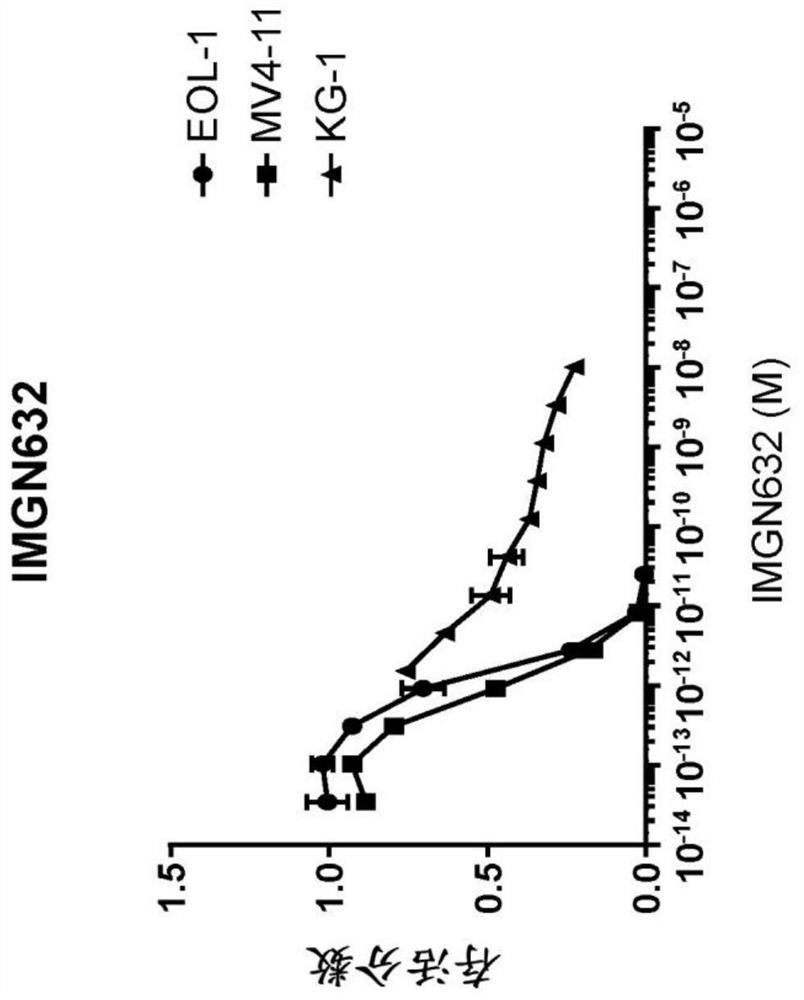
[0321] IMGN632 alone, venetoclax alone and IMGN632 with venetide were studied in in vitro cytotoxicity assays in four different acute myeloid leukemia (AML) cell lines EOL-1, KG-1, Molm-13 and MV4-11 Tork's combined activity.
[0322] EOL-1 , KG-1 , Molm-13 and MV4-11 cell lines were obtained at low passage (less than 10 passages), and cell cultures were maintained as recommended by their suppliers. Cells were harvested from the cell culture in logarithmic growth phase, counted by an automated hemocytometer, and distributed evenly into wells in a 96-well plate such that each well would contain five thousand viable cells / well. Each well contains an Fc receptor blocking, non-mammalian targeting chKTI monoclonal antibody such that the final well volume (200 μL) will contain 100 nM of chKTI. Dosage ranges of IMGN632 alone, venetoclax alone and IMGN632+venetoclax (in the case of venetoclax, t...
Embodiment 2
[0328] Materials and methods for subcutaneous and disseminated xenograft models
[0329] For all subcutaneous xenograft models, mice were weighed twice weekly and clinical signs were monitored throughout the study. Animals were euthanized upon hind leg paralysis, body weight loss greater than 20% of pre-treatment body weight, tumor ulceration, or when any signs of distress were visible.
[0330] Three-dimensionally measure tumor volume in the subcutaneous xenograft model using calipers once to twice a week. The tumor volume uses the formula V=length×width×height×1 / 2 in mm 3 indicated (Tomayko and Reynolds, Cancer Chemother. Pharmacol. 24:148-54 (1989)). Activity was assessed as described in Bissery et al., Cancer Res. 51:4845-52 (1991).
[0331]Tumor growth inhibition (T / C value) was assessed in a subcutaneous xenograft model using the formula: T / C (%) = (median tumor volume treated / median tumor volume control) x 100%. When the tumor volume of the vehicle control reached...
Embodiment 3
[0335] In vivo efficacy of the combination of IMGN632 (single dose) and venetoclax (QDx28) in the EOL-1 subcutaneous model
[0336] To test the efficacy of IMGN632, venetoclax, or the combination of these two agents in their ability to reduce tumor burden in vivo, a subcutaneous tumor model was used as described in the protocol below.
[0337] Female athymic nude mice were each subcutaneously inoculated on the right flank with 1×10 in 100 μl of 50% Matrigel:50% serum-free medium (v / v). 7 EOL-1 cells (human AML cell line). On day 9 (i.e., 24 hours prior to administration of the conjugate for groups receiving conjugate treatment (alone or in combination with venetoclax)), all mice in these treatment groups were injected intraperitoneally with 150 mg / kg of Antibody targeting chKTI to block Fc receptors on EOL-1 AML cells, thereby preventing non-specific uptake of the conjugate. On day 9 after EOL-1 inoculation, mice were randomized into study groups based on tumor volume.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com