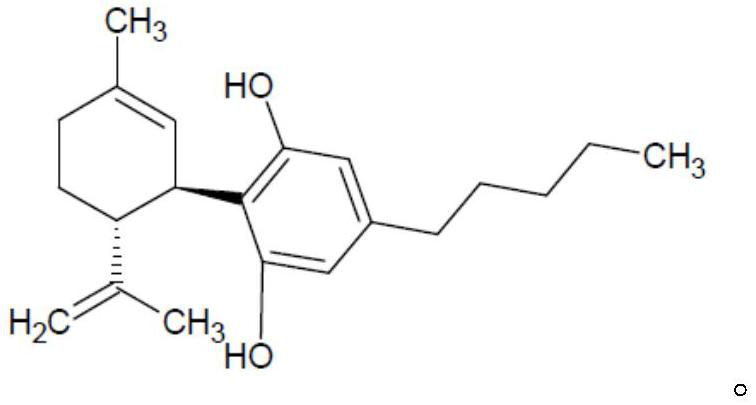
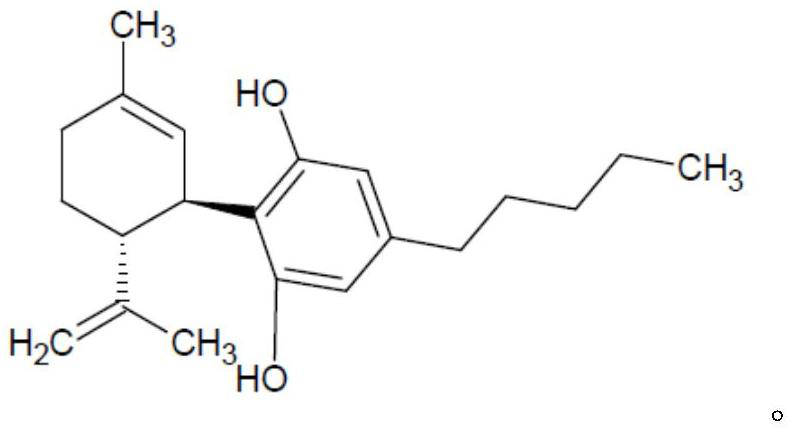
Methods of manufacturing cannabidiol or cannabidivarin and intermediates of manufacturing cannabidiol or cannabidivarin
A technology of cannabidiol and dihydroxy, which is widely used in the field of production of cannabidiol or cannabidiol, and can solve the problems of low total yield, time-consuming and labor-intensive process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
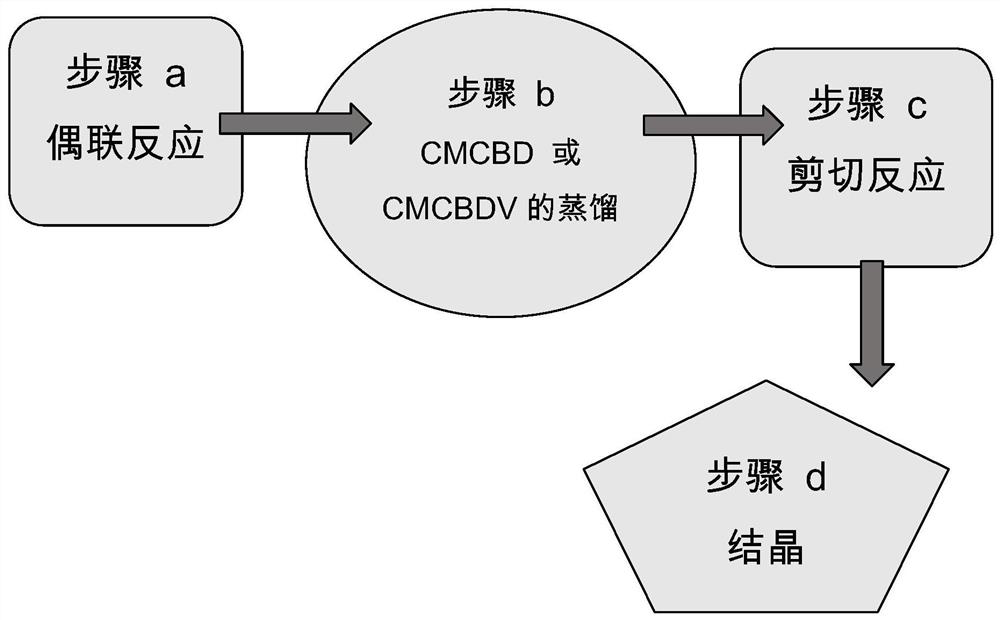
[0118] Preparation of CMCBD ("Coupling Reaction")
[0119] CMCBD was prepared as follows.
[0120] To 3980 mL of dry DCM under nitrogen was added CMO and dissolved. Then add BF 3 , and stirred with CMO for 20 min. PMD in DCM (1:1) was then added rapidly via addition funnel. The reaction was monitored intermittently and finally quenched with an equal volume of saturated bicarbonate before analysis by HPLC.
[0121] The reaction yielded 781.65 grams of CMCBD (molecular weight 372.5) with a nominal purity of 80%.
Embodiment 2
[0123] Preparation of CBD ("shear reaction" )
[0124] CBD is prepared as follows.
[0125] To a 100 L reactor with 15 L of cold water was added 3 kg of NaOH under stirring and nitrogen. An exotherm ensued and the temperature was recorded. Once the temperature was below 65 °C, 15 L of methanol was added. Then, 15 L of methanol containing 8 kg of CMCBD or CMCBDV was added; the reactor was topped up to the 50 liter mark with methanol. Then, with the condenser running, the heat was raised to 95°C. The reaction was monitored periodically (quenched in phosphate buffer and analyzed by HPLC). The reaction takes approximately 24 hours to complete. Upon completion, methanol was removed by distillation (vacuum) and the reaction was allowed to cool. The removed methanol was replaced with the same volume (35 L) of 70:30 hexane:water. With slow stirring, CO 2 Bubble until the water layer is neutral. The organic layer was removed and the aqueous layer was washed with 1 volume of ...
Embodiment 3
[0127] Crystallization of CBD
[0128] CBD was dissolved in hexane at a mass ratio of 1:1 at 50 °C. The solution was then gradually cooled to 20°C, at which point 1% w / w CBD seed mass (>95% purity) was added to a stirred closed vessel. The solution was then allowed to cool to -17°C over 24 hours. The procedure was repeated again using hexane and finally pentane (dissolved in pentane at 35°C, not at 50°C) as above. The crystals were then filtered and the solvent was removed by nitrogen purge or vacuum or both.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com