Application of hydroxychloroquine in medicine for preventing and treating systemic lupus erythematosus
A systemic technology for lupus erythematosus, applied in the biological field, can solve the problems of pathogenic mechanism and effective intervention treatment plan, which have not been reported in literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Comparison of lupus phenotypes between wild-type mice and NCF1 p.R90H gene mutant mice
[0034]Intervention method: Select 10-12 weeks old wild-type mice (WT) and NCF1 p.R90H gene mutant mice (KI), apply 5% imiquimod (IMQ) (2mg / mouse / times), once every other day, for 6-12 weeks, to induce the lupus model. At the same time, for WT and KI mice in the treatment group, hydroxychloroquine (10 mg / kg / day) was administered orally, and the therapeutic effect was detected. The phenotypes of wild-type mice and NCF1 knock-in mice were tested after applying imiquimod cream for 9 weeks.
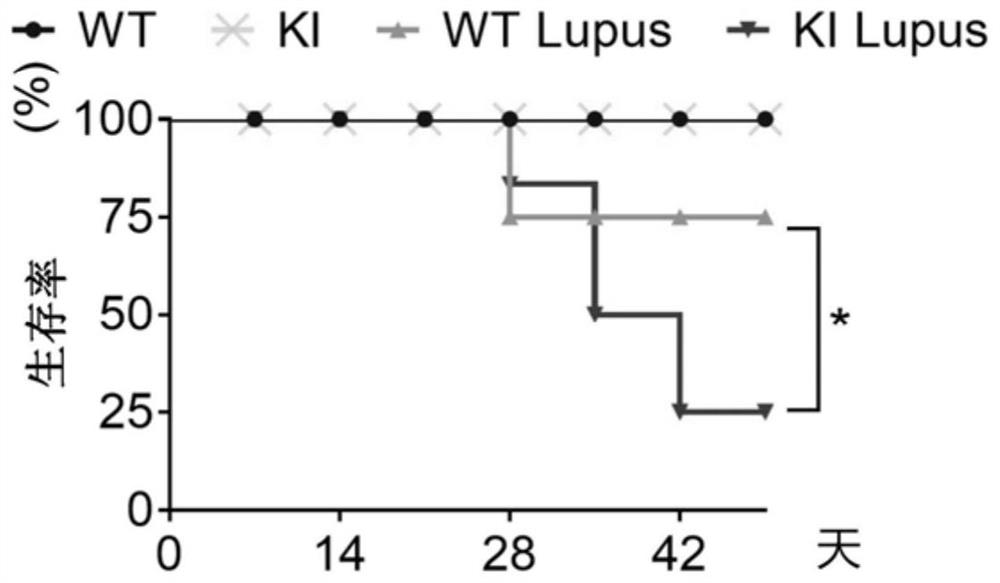
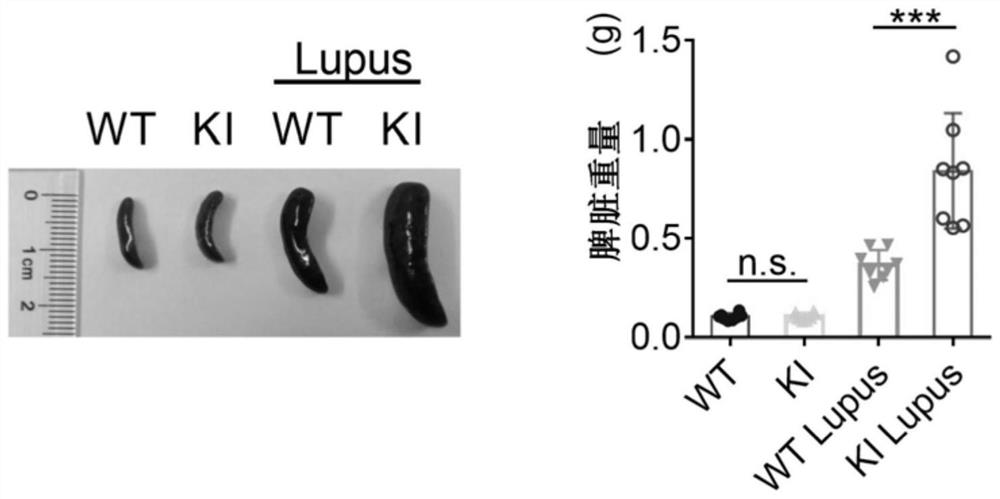
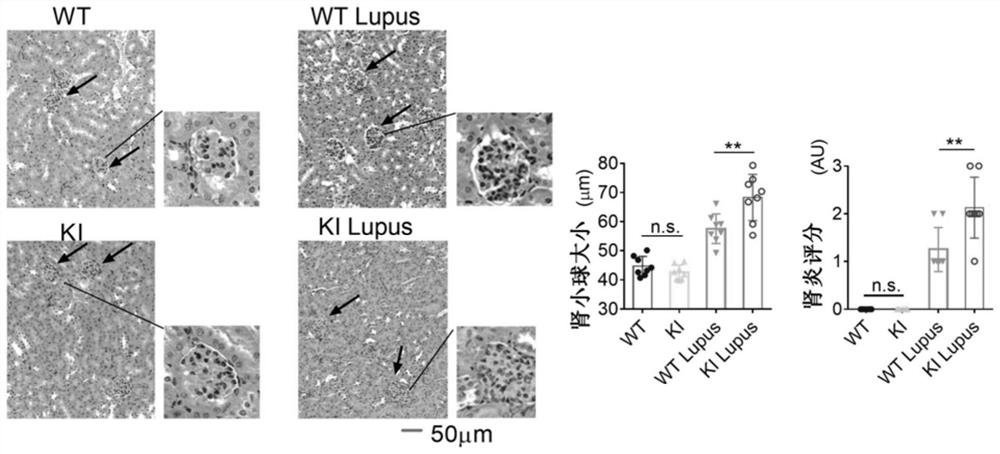
[0035] Four groups of wild type mice (WT), NCF1 p.R90H gene mutant mice (KI), wild type lupus mice (WT+Lupus), NCF1 p.R90H gene mutant lupus mice (KI+Lupus) Experimental results such as figure 1 , 2 , 3 and 4, where figure 1 For survival statistics, figure 2 For spleen pictures and weight comparisons, image 3 Hematoxylin-eosin staining (H&E) pictures of mouse kidneys, glomerulus ...
Embodiment 2
[0041] Example 2 The application of hydroxychloroquine can significantly reduce the lupus symptoms of NCF1 p.R90H gene mutant mice
[0042] Use HCQ to treat wild-type lupus mice (WT Lupus+HCQ) and NCF1 p.R90H gene mutant lupus, and obtain corresponding mouse wild-type lupus mice treatment group (WT Lupus+HCQ), NCF1 p.R90H gene mutation Type lupus mouse treatment group. And wild-type lupus mice (WT+Lupus), NCF1 p.R90H gene mutant lupus mice (KI+Lupus), wild-type lupus mice treatment group (WT Lupus+HCQ), NCF1 p.R90H gene mutant The phenotypes of the lupus mouse treatment group (KI Lupus+HCQ) were detected and compared. Test results such as Figure 5 , 6 , 7 and 8. The wild-type mice and the NCF1 knock-in mice were given imiquimod cream for 7 weeks at the same time.
[0043] like Figure 5 As shown, wild-type lupus mice (WT+Lupus), NCF1 p.R90H gene mutant lupus mice (KI+Lupus), wild-type lupus mice treatment group (WT Lupus+HCQ), NCF1 p.R90H gene mutation Survival statist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com