Gene editing system and method for fixed-point insertion of exogenous gene
An exogenous gene and gene editing technology, applied in the field of biomedicine, can solve problems such as copy number and position instability, interference with experimental results, and pathogenicity. Simple and uniform background, clear and reliable effect of genetic background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Design and synthesis of exogenous genes and sgRNA
[0043] 1. Design of sgRNA
[0044] Using CRISPR / Cas9 technology, for the AAVS1 region, use the Crisprgold website (https: / / crisprgold.mdc-berlin.de / index.php) to design sgRNA, the selected sgRNA sequence is shown in SEQ ID NO: 1 (GGGGCCACTAGGGACAGGAT).
[0045] 2. Target genome amplification
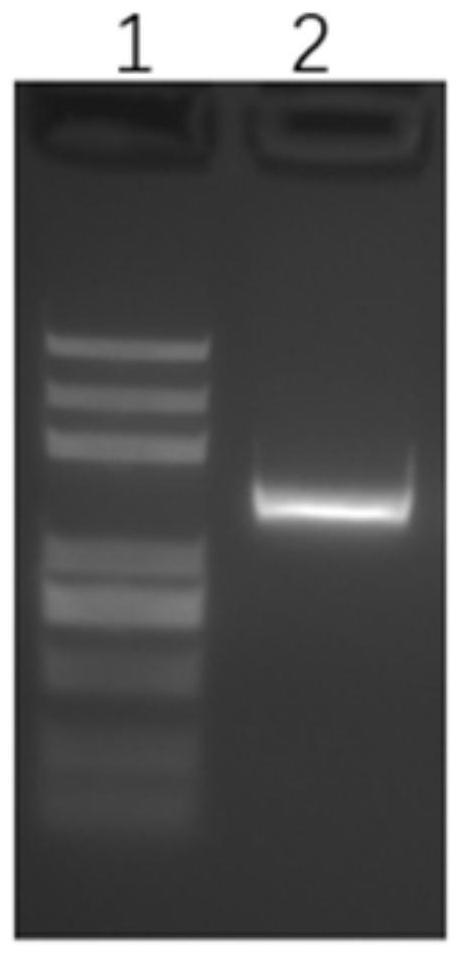
[0046] Take 1×10 6 Genomic DNA was extracted using Tiangen Cell Gene Extraction Kit (DP304), and genomic PCR amplification was performed using primers AAVS1-F1 (SEQ ID NO:5, GCCTCCCCTTCTTGTAGGCC) and AAVS1-R1 (SEQ ID NO:6, AGCCAAAGTTAGAACTCAGG) Then use the gel kit (Axygen: AP-GX-250) to cut the amplified fragment and recover the target fragment. The result is as follows: figure 1 shown, and the recovered target fragments were stored at -20°C until use.
[0047] 3. Preparation of AAVS1 insertion site sgRNA in vitro and verification of editing efficiency
[0048] sgRNA in vitro transcription: according to the selec...
Embodiment 2
[0052] Example 2: Site-directed insertion of exogenous genes into K562 cells
[0053] 1. K562 cell recovery culture
[0054] Turn on the water bath in advance, and set the temperature to 37°C, take out the IMDM-Full medium to preheat, take out the NK-92 cells from the liquid nitrogen tank, put them into the 37°C water bath immediately, take a 15ml centrifuge tube, add 8ml pre-heated Warmed K562 medium; place the cells in a 37°C water bath and shake gently until they are completely melted, wipe the outer wall of the cryovial with an alcohol-containing non-woven cloth, and slowly add the cell suspension to a 15ml centrifuge tube (this The process is controlled within 3 minutes), after mixing, centrifuge at 1000rpm for 5 minutes, discard the supernatant; take 1ml of medium to resuspend the cell pellet, transfer to a T75 culture flask containing 30mL IMDM-Full medium for culture, and carry out cell passage according to a certain density. Maintain proper cell growth density.
[0...
Embodiment 3
[0059] Example 3: Detection of electroporation efficiency of aAPC1 knock-in K562 cells
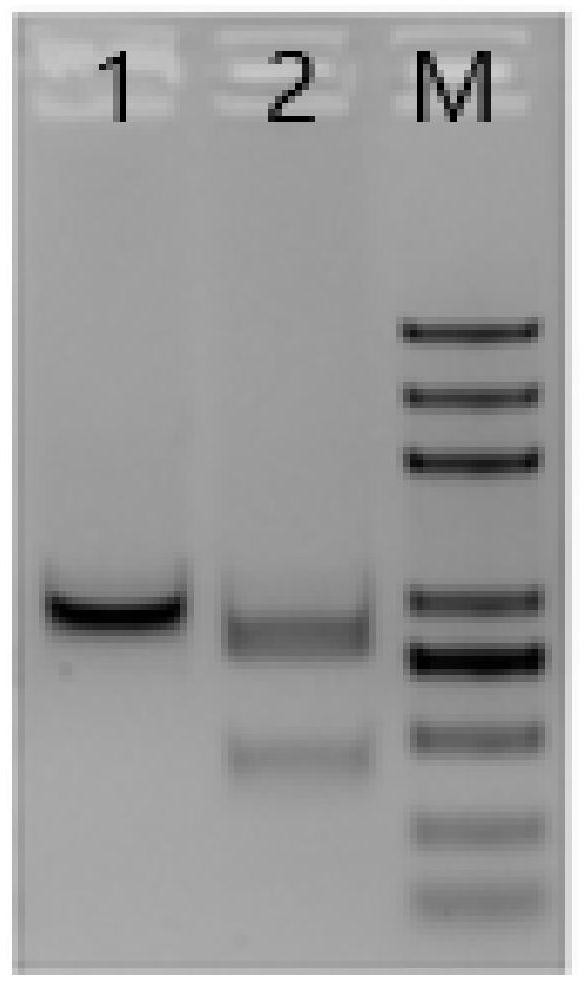
[0060] Take part of the K562 cells with aAPC1 knock-in after transfection for 24 hours in Example 2, transfer to a 15ml centrifuge tube, centrifuge at 1500rpm for 5min, discard the supernatant; add 1ml PBS to wash the cell pellet, transfer to a 1.5ml EP tube, Centrifuge at 1500rpm for 5min, discard the supernatant, repeat the operation once, use the flow cytometer (ACEA:NOVOCyte3130), select the FITC channel to detect the electroporation efficiency, the results are as follows Figure 4 As shown, it can be seen from the figure that the RNP complex electrotransfected K562 cells has a transfection efficiency of 49.71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com