Nucleation cryopreservation method of immune cells
A cryopreservation method and technology of immune cells, applied in the field of cell biology, can solve the problems of lack of rapid cryopreservation methods for immune cells, etc., to solve the problem of uncontrollable sample cryopreservation process, good cryopreservation effect, and little damage to cells or human body Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
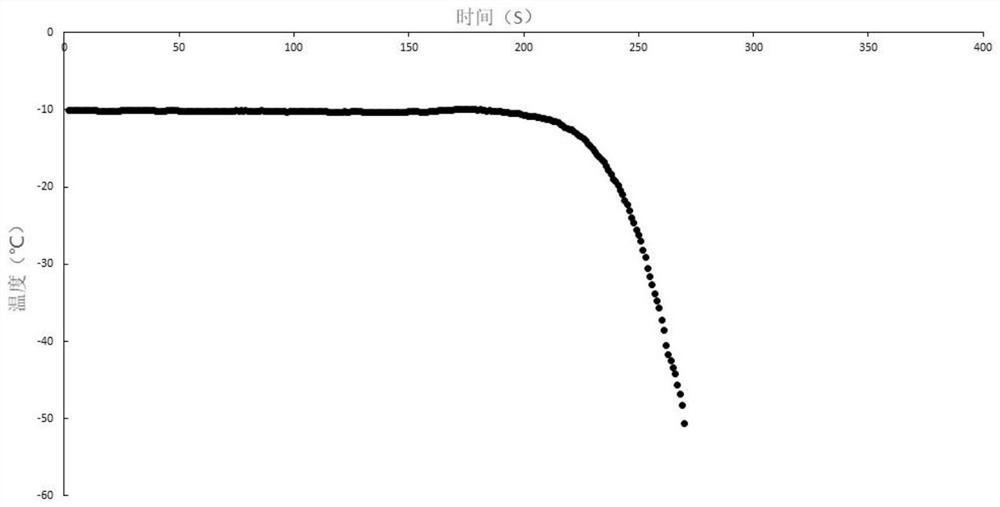
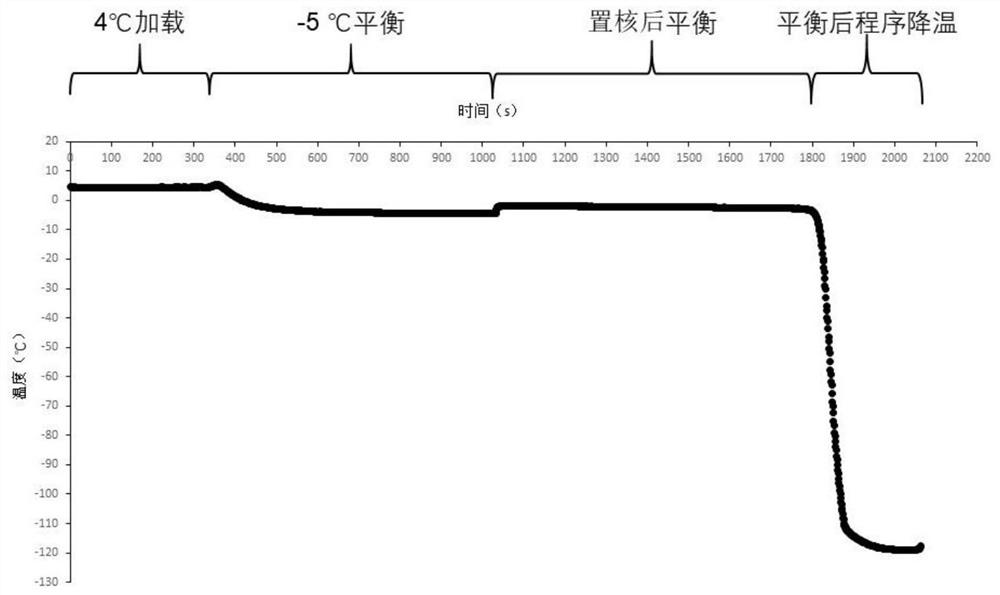
[0058] The specific process of nuclear freezing of human T lymphocytes, the experimental results of 50℃ / min programmed cooling after nucleation at -5℃ and -10℃.
[0059] 1. Preparation of cryopreservation medium for human T lymphocytes
[0060] 13.692g trehalose (Sinopharm Chemical Reagent Co., Ltd.) was dissolved with 56ml physiological saline, and mixed; then slowly added 40ml human albumin injection (GRIFOLS, 10g (20%: 50ml)), 4ml ethylene glycol (Sinopharm Chemical Reagent Co., Ltd.), mix well, and store in a 4°C refrigerator for later use. Therefore, the formula of the 2×cell cryopreservation solution in this embodiment is: 8% HSA, 4% ethylene glycol, and 0.4M trehalose.
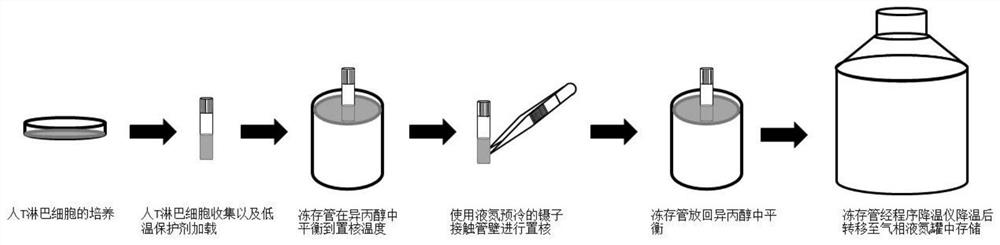
[0061] 2. Collection of Human T Lymphocytes and Loading of Protective Agents
[0062] Transfer the expanded and cultured human T lymphocyte suspension to a 50ml centrifuge tube and take 50μl for counting, balance and place in a centrifuge with a centrifugal force of 500g and a centrifugation time of 5...
Embodiment 2
[0070] In this embodiment, the survival rate and recovery rate of the cells are the results when the cells have just recovered. The survival rate is directly obtained from the Cellometer Auto 2000 cell counter (NexcelomBioscience, Lawrence, USA). The formula for calculating the recovery rate is as follows:
[0071] Cell recovery rate = viable cell concentration before cryopreservation / viable cell concentration after cryopreservation.
[0072] Determination of the best range of cooling rate after nuclear setting
[0073] 1. Preparation of cryopreservation medium for human T lymphocytes
[0074] 10.269g trehalose (Sinopharm Chemical Reagent Co., Ltd.) was dissolved with 20ml of normal saline, mixed evenly, and settled to a final volume of 30ml, and prepared into 1M trehalose mother liquor; The ratio is 2× freezing solution, and it is stored in a refrigerator at 4°C for later use.
[0075] 2. Collection of Human T Lymphocytes and Loading of Protective Agents
[0076] Transfer ...
Embodiment 3
[0084] Determination of the optimum range of core temperature
[0085] 1. Preparation of cryopreservation medium for human T lymphocytes
[0086] 10.269g trehalose (Sinopharm Chemical Reagent Co., Ltd.) was dissolved with 20ml of normal saline, mixed evenly, and settled to a final volume of 30ml, and prepared into 1M trehalose mother liquor; The ratio is 2× freezing solution, and it is stored in a refrigerator at 4°C for later use.
[0087] 2. Collection of Human T Lymphocytes and Loading of Protective Agents
[0088] Transfer the expanded and cultured human T lymphocyte suspension to a 50ml centrifuge tube and take 50μl for counting, balance and place in a centrifuge with a centrifugal force of 500g and a centrifugation time of 5min. Resuspend the cells with 1640 medium and determine the amount of 1640 medium to be added according to the counting results, and adjust the cell density to 1×10 7 A / ml or so. After adjusting the cell density, centrifuge again, the centrifugal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com