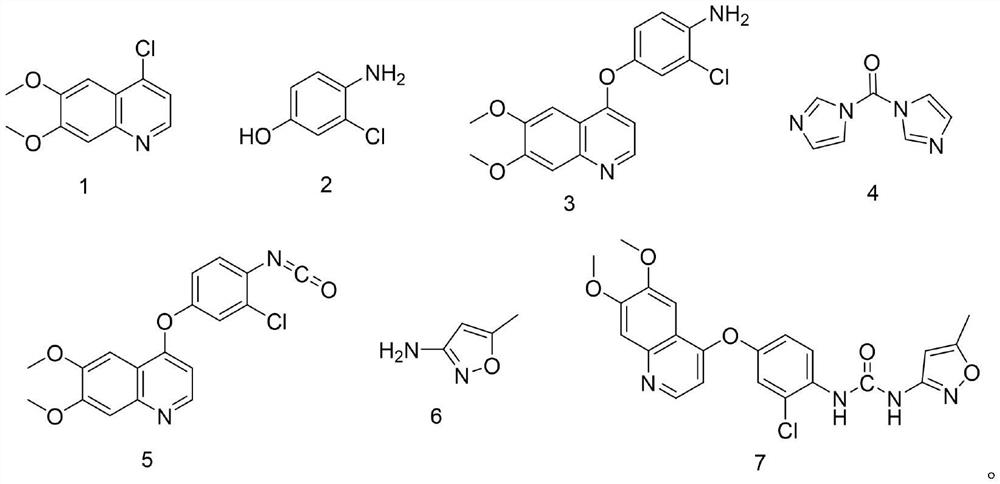
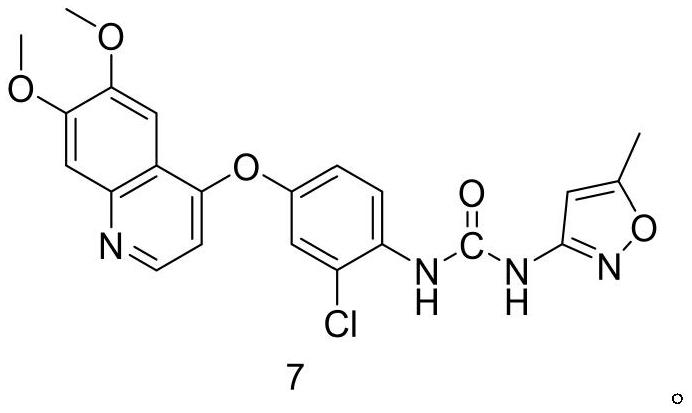
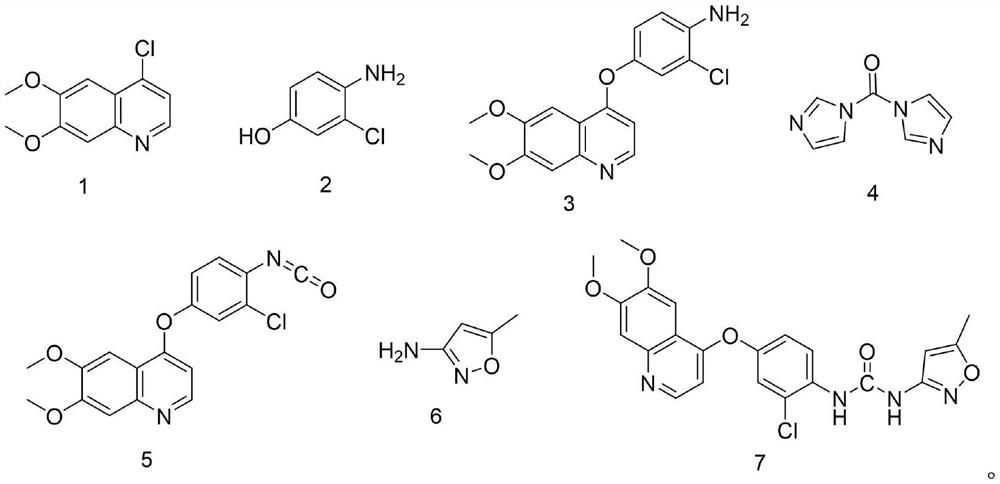
Synthesis process of VEGFR inhibitor tevozanib
A technology for tivozanib and synthesis process, which is applied in the field of biomedicine, can solve problems to be improved, etc., and achieves the effects of high yield, simplified reaction route and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7
[0054] Embodiment 7 is a comparative example for preparing the compound shown in formula 5. In this example, the inventor adjusted the reaction molar ratio of the compound shown in formula 3 to the compound shown in formula 4 to be 1:1.6, and adjusted the compound shown in formula 3 The reaction time with the compound shown in formula 4 is 5 hours, and on its technical effect, the product yield that the present embodiment obtains is 1:(1.05~ 1.4), and the reaction time of the compound shown in formula 3 and the compound shown in formula 4 contact stirring is the product yield when 2~3 hours, does not obtain obvious improvement, therefore, from the point of view of material consumption and energy consumption, the present invention The molar ratio of the compound shown in the formula 3 and the compound shown in the formula 4 in the step (2) is 1: (1.05~1.4), and the reaction time of the compound shown in the formula 3 and the compound shown in the formula 4 is 2~3 hours, thus no...
Embodiment 11
[0064] Example 11 is a comparative example for preparing the compound shown in formula 7. In this example, the inventor adjusted the reaction molar ratio of the compound shown in formula 5 to the compound shown in formula 6 to be 1:1.6, and adjusted the compound shown in formula 5 The reaction time with the compound shown in formula 6 is 4 hours, and on its technical effect, the product yield that the present embodiment obtains is 1:(1.0~ 1.3), and the product yield when the compound shown in formula 5 and the compound shown in formula 6 are contacted and stirred for 1 hour 45 minutes~2.5 hours, the product yield has not been improved, on the contrary the product yield has decreased, in addition, obtained The HPLC purity of the compound shown in formula 7 was significantly reduced from 99.7% to 98.2%, resulting in increased product impurities. Therefore, from the perspective of material consumption and energy consumption, the molar ratio of the compound shown in formula 5 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com