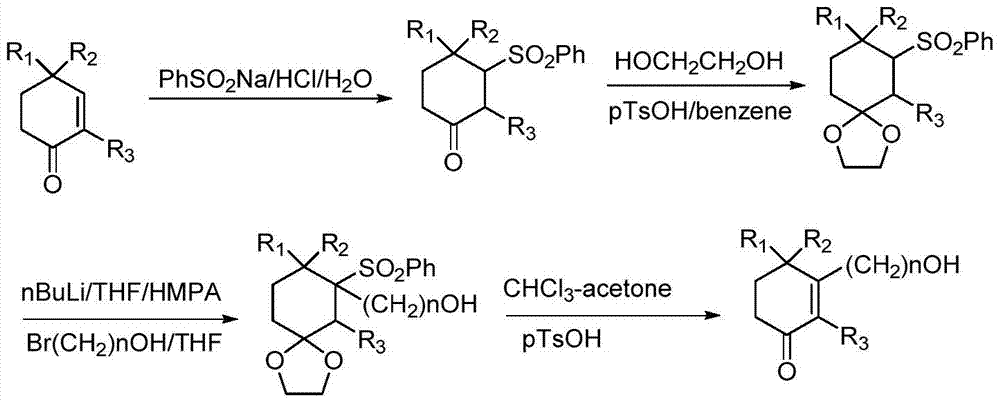
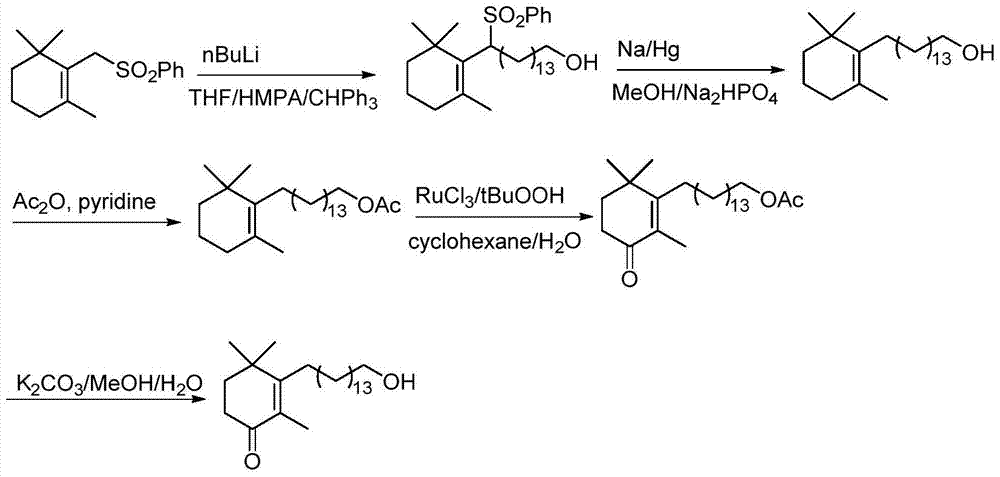
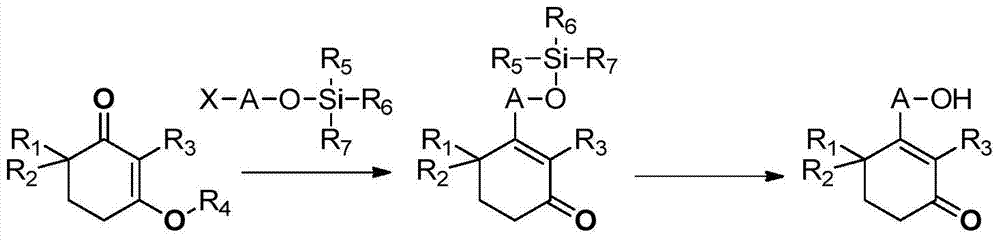
Preparation method of high-purity cyclohexenone long-chain alcohol
A technology of cyclohexenone long-chain alcohol and cyclohexenone, which is applied in the field of medicinal chemistry and synthetic chemistry, and can solve the problems of high cost, large loss and low melting point of column chromatography
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 13
[0085] Preparation 13-isobutoxy-2,6,6-trimethylcyclohex-2-en-1-one
[0086]
[0087] 2,4,4-trimethylcyclohexyl-1,3-dione VII (80g, 1eq) and isobutanol (76.9g, 2eq) were added to cyclohexane (400mL), and p-TSA. h 2 O (5g, 0.05eq), heated to reflux to separate water for 16h. Post-treatment, cooled to ambient temperature, washed successively with 5% sodium hydroxide (80mL), water (80mL) and saturated brine (80mL), dried over anhydrous sodium sulfate, concentrated to dryness to obtain 3-isobutoxy-2 , 6,6-Trimethylcyclohex-2-en-1-one (103.65 g, 95%). 1H NMR (400MHz, CDCl3): δ3.77(d, 2H, J=6.4Hz), 2.55-2.58(m, 2H), 1.95-2.05(m, 1H), 1.82(t, 2H, J=6.4Hz ), 1.72(s, 3H), 1.11(s, 6H), 1.01(d, 6H, J=6.4Hz).
[0088] Preparation 23-Cyclohexylmethoxy-2,6,6-trimethylcyclohex-2-en-1-one
[0089]
[0090] 2,4,4-trimethylcyclohexyl-1,3-dione VII (10g, 1eq) and cyclohexanemethanol (14.8g, 2eq) were added to cyclohexane (100mL), and p-TSA· H2O (0.62g, 0.05eq), heated to reflux to sep...
preparation example 43-
[0094] Preparation 43-Methoxy-2,6,6-trimethylcyclohex-2-en-1-one
[0095]
[0096] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (2.7g, 1eq) and trimethyl orthoformate (2.8g, 1.5eq) were added to methanol (40mL), p- TSA·H2O (167mg, 0.05eq), stirred overnight at room temperature. After treatment, add dichloromethane (30mL) to dilute, wash with 5% sodium hydroxide (20mL), water (10mL) and saturated brine (10mL) successively, dry over anhydrous sodium sulfate, concentrate to dryness, and perform column purification to obtain 3 - Methoxy-2,6,6-trimethylcyclohex-2-en-1-one (2.19 g, 74.4%). 1 H NMR (400MHz, CDCl 3 ):δ3.81(s,3H),2.55-2.58(m,2H),1.95-2.05(m,1H),1.82(t,2H,J=6.4Hz),1.72(s,3H),1.11( s,6H).
[0097] Preparation 53,3'-(Propyl-1,2-dioxo)-bis(2,6,6-trimethylcyclohexyl-2-en-1-one)
[0098]
[0099] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (5g, 1eq), 1,3-propanediol (1.23g, 0.5eq), p-TSA·H2O (311mg, 0.05eq) And toluene (30mL) was added to the flask, heated to reflux to se...
preparation example 93-(1
[0110] Preparation 9 3-(15-chloropentadecyloxy)-2,6,6-trimethylcyclohexyl-2-en-1-one
[0111]
[0112] 2,4,4-Trimethylcyclohexyl-1,3-dione VII (1.3 g, 1.1 eq) and 15-chloropentadecanol VIII-1 (2 g, 1 eq) were added to cyclohexane (50 mL) Add p-TSA·H2O (72mg, 0.05eq), heat to reflux to separate water for 16h, post-treatment, cool to ambient temperature, successively wash with 5% sodium hydroxide (20mL), water (10mL) and saturated saline ( 10mL) was washed, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 3-(15-chloropentadecyloxy)-2,6,6-trimethylcyclohexyl-2-en-1-one (2.46g, 80.9 %). 1 H NMR (400MHz, CDCl 3 ):δ3.97(t,2H,J=6.8Hz),3.45(m,2H,J=6.8Hz),2.54-2.55(m,2H),1.78-1.84(m,4H),1.68(s, 3H),1.39-1.41(m,4H),1.22-1.35(m,21H),1.08(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com