3-(3-(heteroaryl)-4-thiazolinone)-N-aryl benzamide compound and synthesis and application thereof
A technology of aryl benzamide and thiazolinone, which is applied in the field of biomedicine and synthetic medicine to achieve good inhibitory and selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
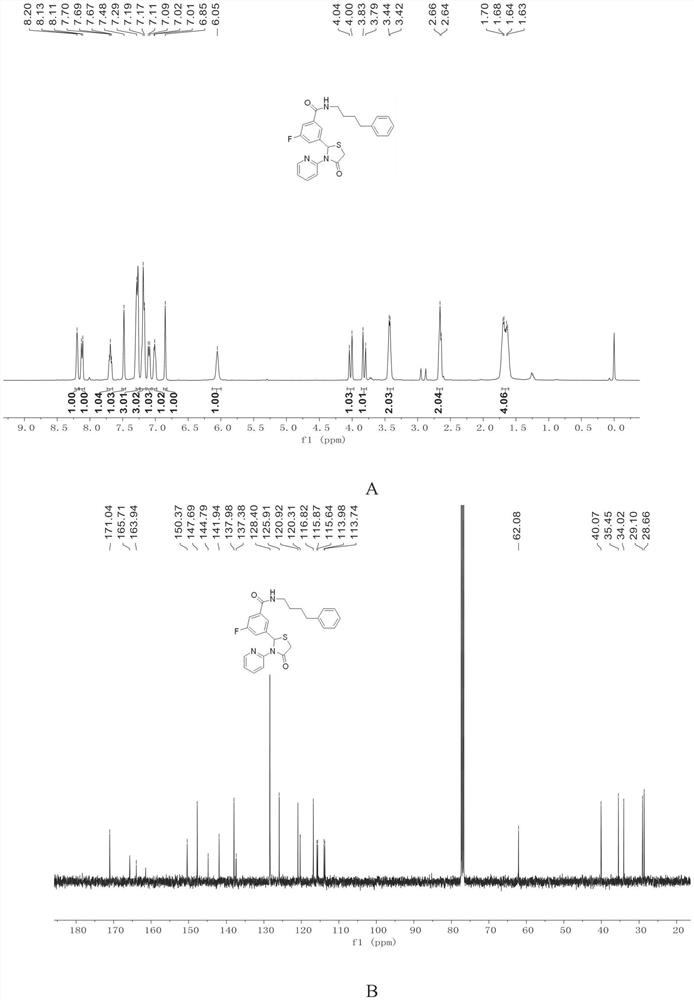
[0091]
[0092] III-1 is a foamy white solid with a yield of 92%.
[0093] 1 H NMR (400MHz, Chloroform-d) δ8.20(s, 1H), 8.12(d, J=8.3Hz, 1H), 7.69(t, J=6.9Hz, 1H), 7.48(s, 1H), 7.29 (s,3H),7.18(d,J=7.1Hz,3H),7.10(d,J=8.6Hz,1H),7.02(d,J=4.6Hz,1H),6.85(s,1H),6.05 (s,1H),4.02(d,J=16.1Hz,1H),3.81(d,J=16.1Hz,1H),3.43(d,J=5.7Hz,2H),2.65(d,J=7.2Hz ,3H),1.79–1.50(m,6H).
[0094] 13 C NMR (101MHz, CDCl 3 )δ171.04,165.71,163.94,150.37,147.69,144.79,141.94,137.98,137.38,128.40,125.91,120.92,120.31,116.82,115.87,115.64,113.98,113.74,62.08,40.07,35.45,34.02,29.10,28.66.
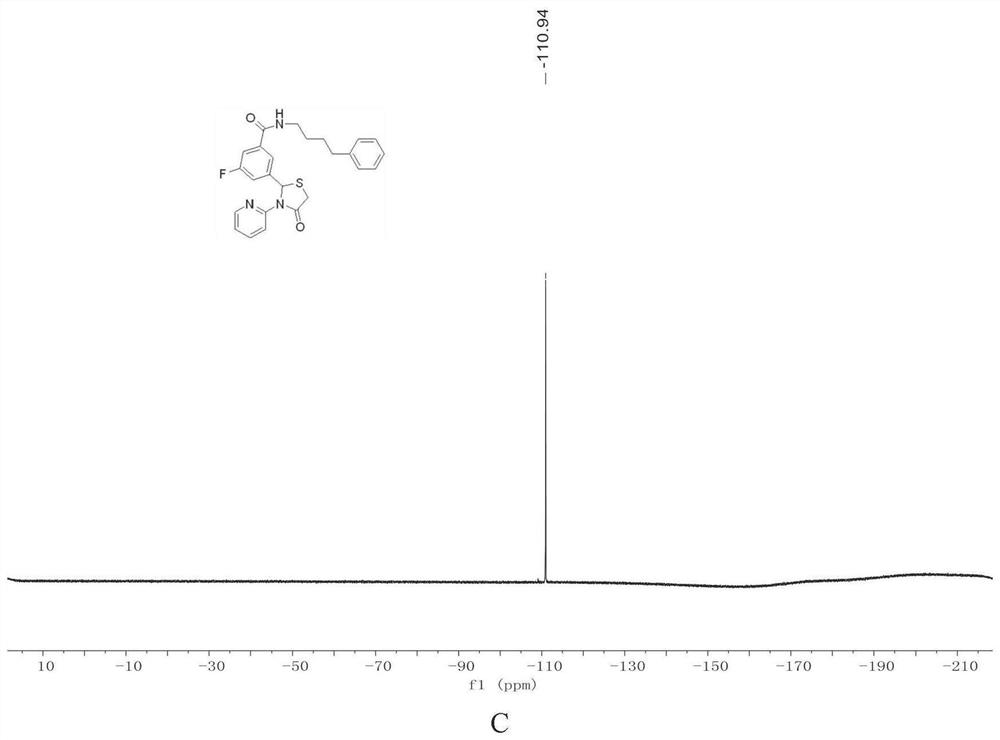
[0095] 19 F NMR (376MHz, CDCl 3 )δ-110.94.
Embodiment 2
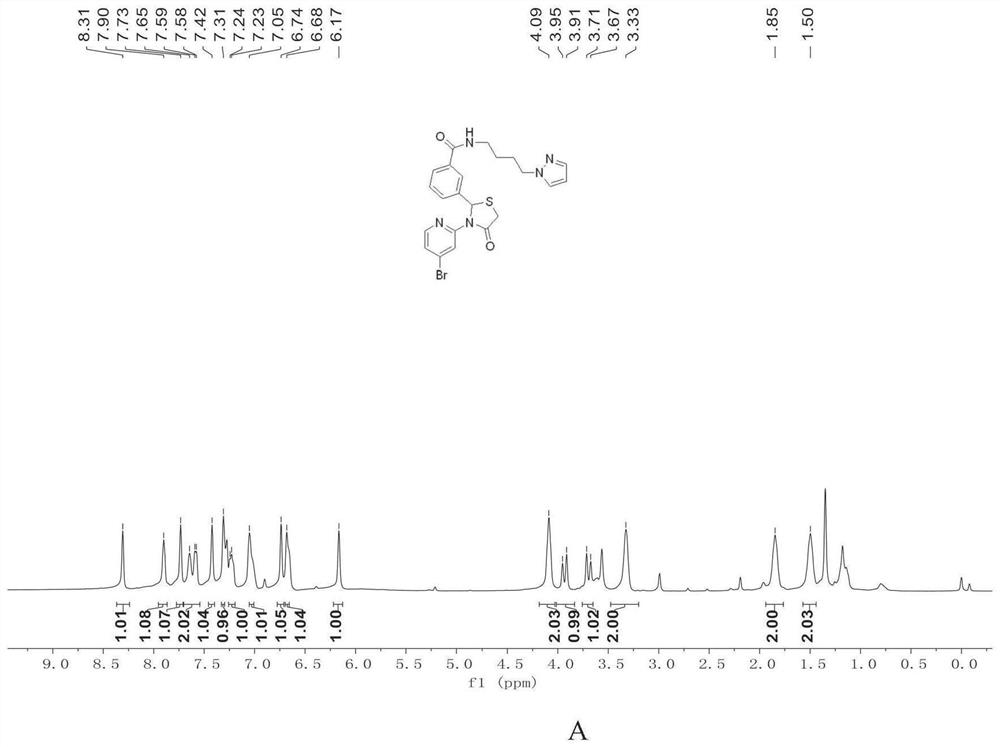
[0097]
[0098] Yield = 65%.
[0099] NMR spectrum:
[0100] 1 H NMR (400MHz, Chloroform-d) δ8.31(s,1H),7.90(s,1H),7.73(s,1H),7.67–7.55(m,3H),7.42(s,1H),7.31( s,2H),7.26–7.20(m,2H),7.05(s,2H),6.74(s,1H),6.68(s,2H),6.17(s,1H),4.09(s,3H),3.93 (d,J=16.1Hz,1H),3.69(d,J=16.0Hz,1H),3.33(s,2H),1.85(s,2H),1.50(s,3H).
[0101] 13 C NMR (101MHz, CDCl 3 )δ171.35,167.05,158.46,151.16,148.06,145.39,141.66,139.12,135.15,133.89,129.35,128.89,128.55,126.77,125.02,124.01,119.56,116.59,112.65,105.54,62.57,51.27,39.58,33.97,30.32 ,27.98,26.08.
Embodiment 3
[0103]
[0104] Yield = 81%.
[0105] NMR spectrum:
[0106] 1 HNMR(400MHz,Chloroform-d)δ8.16(s,1H),7.98(s,1H),7.73(s,1H),7.58(d,J=6.3Hz,1H),7.43(s,1H), 7.36–7.16(m,4H),6.90(s,2H),6.75(s,1H),6.17(s,1H),4.10(s,2H),3.93(d,J=16.2Hz,1H),3.70 (d,J=16.2Hz,1H),3.35–3.24(m,3H),1.86(s,2H),1.50(s,2H).
[0107] 13 C NMR (101MHz, CDCl 3 )δ171.34,166.98,151.35,148.17,145.35,141.72,139.18,135.18,129.30,128.51,126.70,125.01,121.10,116.63,105.54,62.58,51.26,39.59,33.98,30.32,29.69,28.01,26.05.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com