Transgenic animal production method and rabies animal model construction method
A technology of transgenic animals and production methods, which is applied in the field of transgenic animal production methods and rabies animal model construction, can solve problems such as raising the threshold of rabies research, and achieve the effect of improving biological safety and lowering the research threshold
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0068] Example 2 Transgenic mouse construction
[0069] C57BL / 6 mice were used as experimental animals.
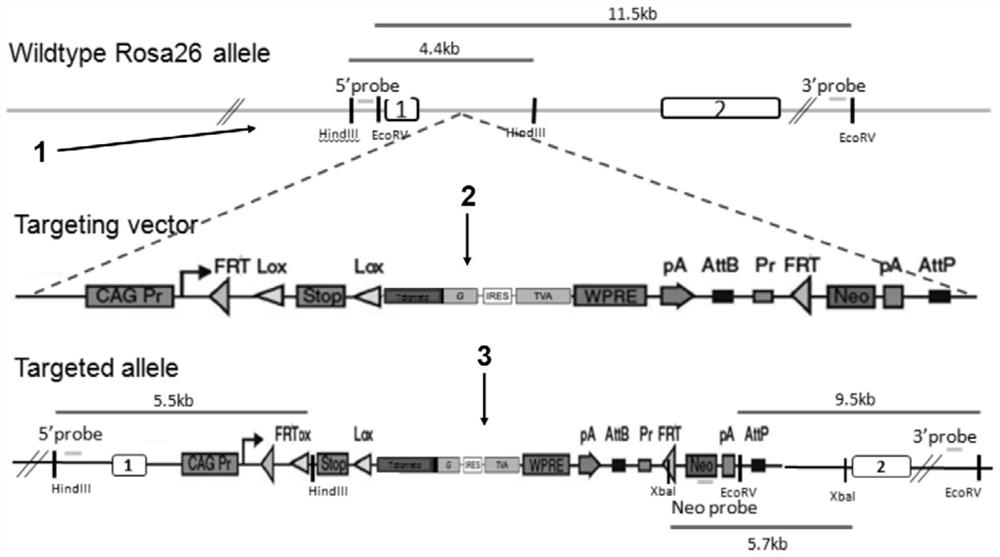
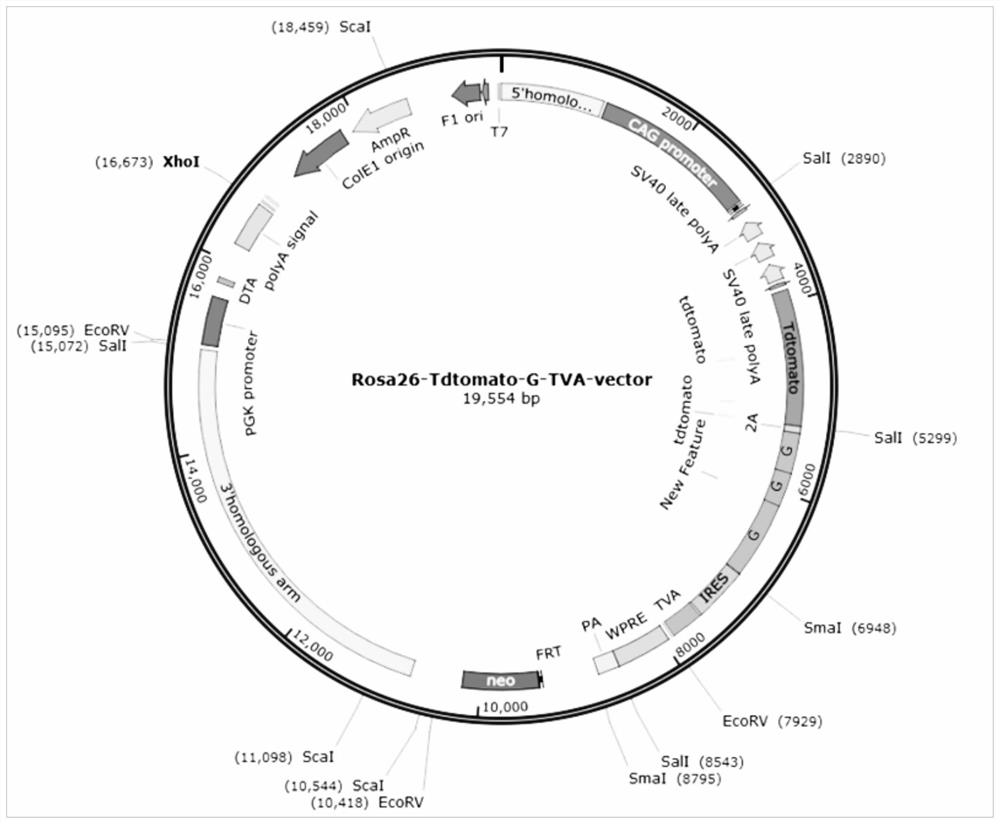
[0070] The targeting vector was linearized, and then ES cells (Embryonic stem cells, embryonic stem cells, hereinafter referred to as ES cells) of C57BL / 6 mice were electroporated. Screen correctly recombined ES cell clones (i.e. ES cell clones that successfully target).
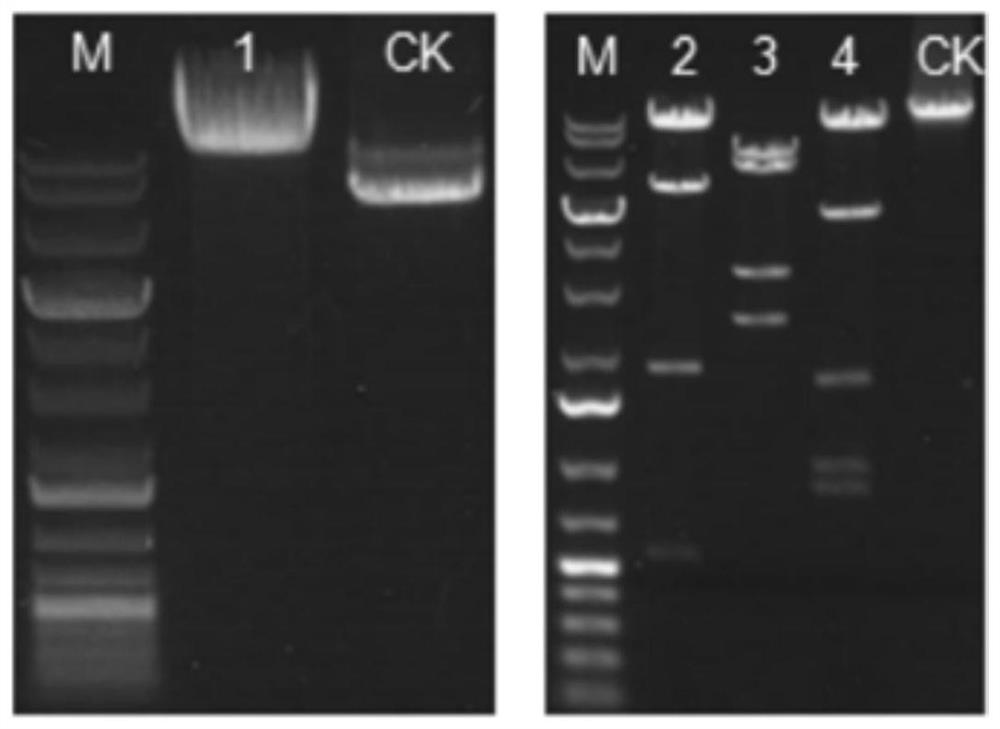
[0071] Screening strategy: Select the Southern blot method, and use 5'Probe (probe), 3'Probe and NeoProbe to screen ES cells at the same time, and introduce a 5' Southern restriction site between the 5' loxP site and the 3×Stop element point, there is a 3' Southern restriction site downstream of the Neo cassette, if correct recombination occurs, there will be two bands of wild type and mutant type; if not correct recombination, only the wild type band will appear.
[0072] restriction endonuclease Probe Wild type (WT) amplification results Mutant (Targeted) amplification results Hi...
Embodiment 3
[0091] Embodiment 3 Rabies animal model construction
[0092] The GT mice capable of expressing the G protein constructed in Example 2 were used as infected animals, and the G protein-deficient CVS-N2C strain RV was used as the infection strain to inoculate and infect GT mice (intranasally or intramuscularly).
[0093] Such as Figure 9 Shown, 1 is the original genotype of this virus strain, carries N, P, M, G, the coding gene of five kinds of viral proteins of L. 2 is the genotype after the strain lost the G protein, and the G protein was replaced by GFP (green fluorescent protein).
[0094] After infection, any infected cell in GT mice provides G protein to G protein-deficient CVS-N2C strain RV, restores the infection and transmission activity of street virus RV, simulates the natural infection process of RV street virus, and successfully constructs rabies animals Model.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com