Application of doxepin hydrochloride in preparation of antiviral drugs
A technology of doxepin hydrochloride and virus, which is applied in the field of medicine, can solve the problem of doxepin hydrochloride without doxepin, and achieve the effect of inhibiting virus replication
Active Publication Date: 2022-04-15
SHENZHEN TECH UNIV
View PDF16 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
But so far, there is no relevant research on the application of doxapine hydrochloride in the preparation of anti-Coxsackievirus B virus drugs in the prior art
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
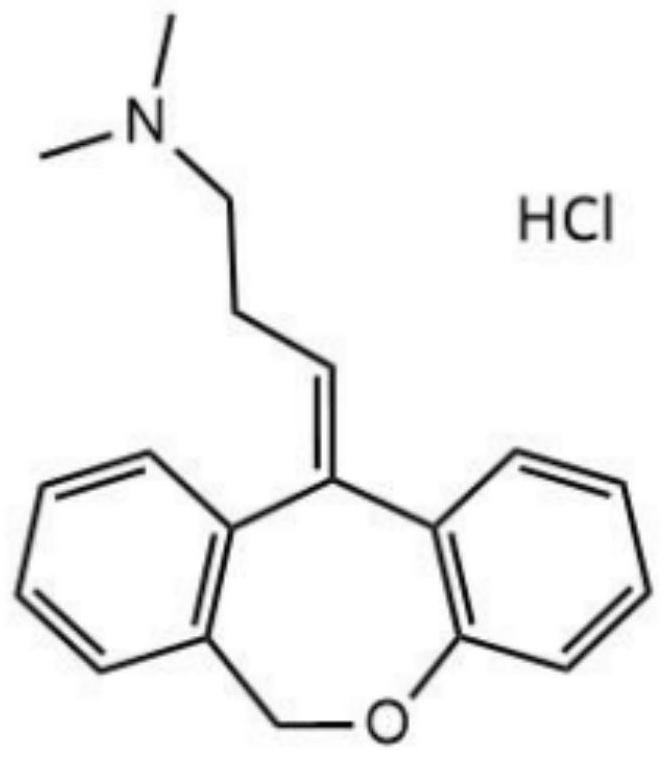
[0038] The chemical structural formula of doxepin hydrochloride is as figure 1 shown.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
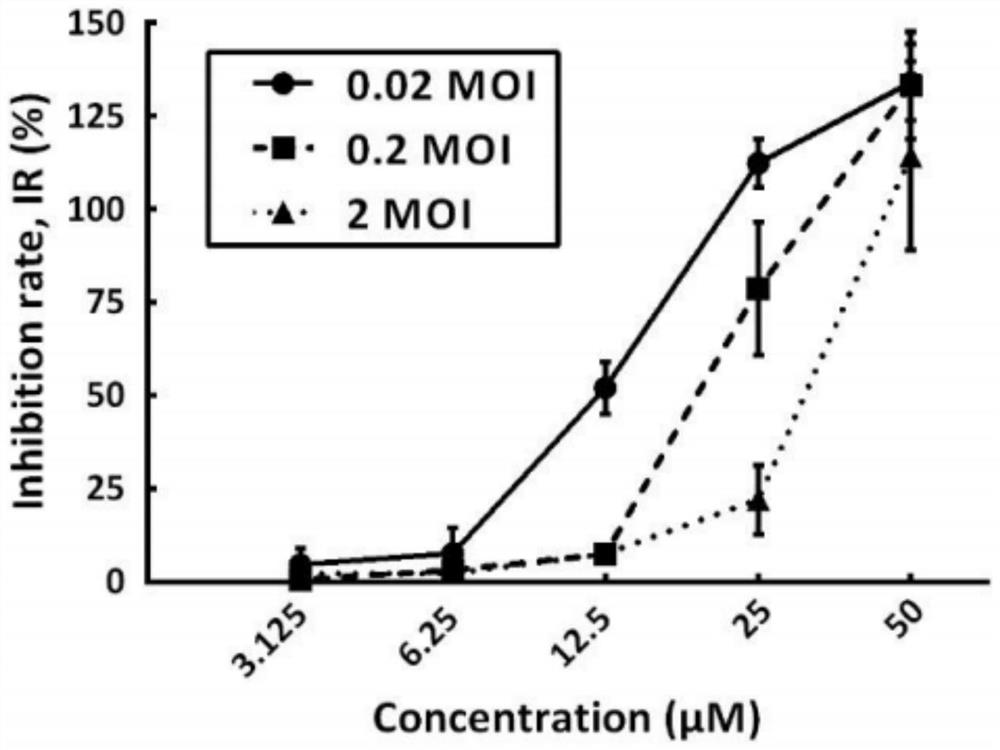
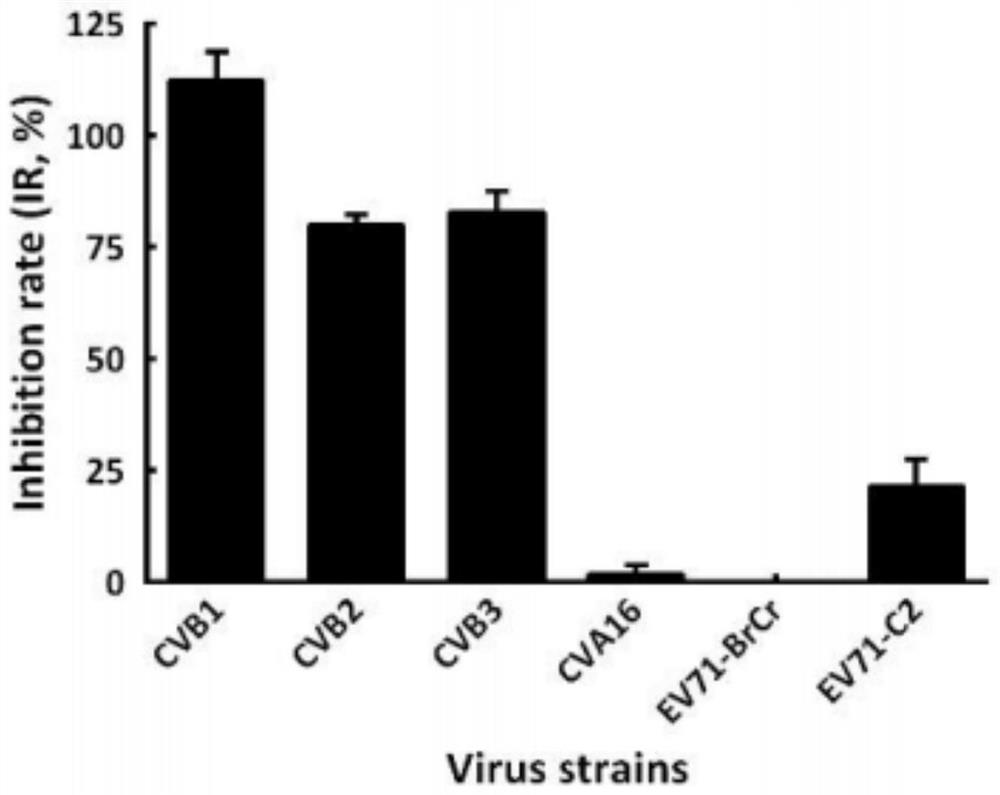
The invention provides an application of doxepin hydrochloride in preparation of antiviral drugs, and relates to the technical field of medicines, research shows that doxepin hydrochloride shows relatively strong antiviral activity to coxsackie B viruses type 1, type 2 and type 3, the minimum 50% inhibitory concentration (IC50) value is 10.12 + / -0.85 mu M. In addition, it is found that doxepin hydrochloride inhibits virus replication in the early stage of an infection cycle, and doxepin hydrochloride can inhibit virus replication in the early stage of the infection cycle. Meanwhile, some host genes related to a mechanism are found through target spot prediction and gene association network analysis, and foundation and basis are provided for doxepin hydrochloride used for treating coxsackie type B virus infection.
Description
technical field [0001] The invention relates to the technical field of medicine, in particular to the application of doxepin hydrochloride in the preparation of antiviral drugs. Background technique [0002] Coxsackievirus B (CVB) is a subgroup of human enterovirus B (HEV-B), belonging to the family Picornaviridae, including six serotypes: B1 to B6. CVB is generally recognized as the causative agent of human cardiac and muscular diseases, especially myocarditis, in addition, other inflammatory diseases such as aseptic meningitis, pleuritic pain, panuveitis, acute pancreatitis and severe hepatitis have been reported to be associated with CVBs infection related. In recent years, there has been some evidence that CVB can also cause hand, foot and mouth disease (HFMD). The commonly treated subtypes are B3 and B5, whose associated medical records can be found in children and adults, and other cases induced by B1, B2 and B4 are also sporadically distributed. Coxsackie B virus b...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/335A61P31/14
CPCY02A50/30A61K31/335A61P31/14
Inventor 朱钦昌杨咏祺刘格贺震旦
Owner SHENZHEN TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com