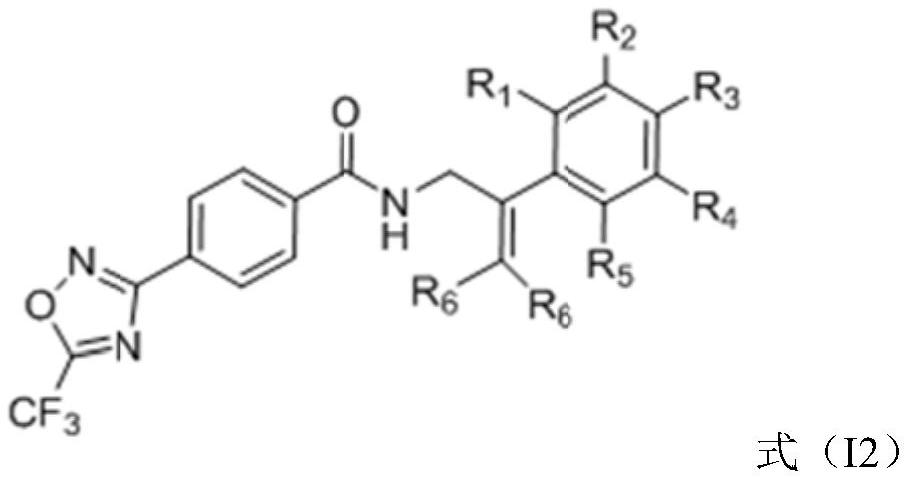
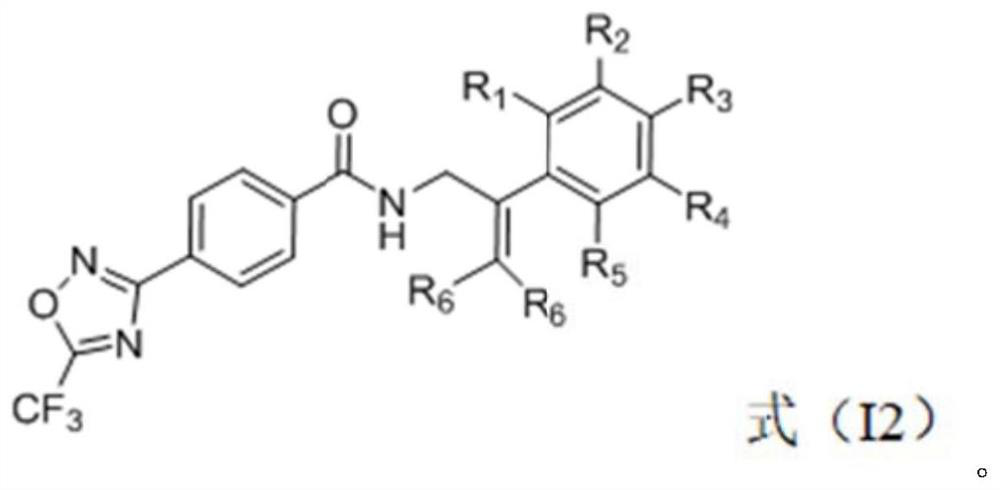
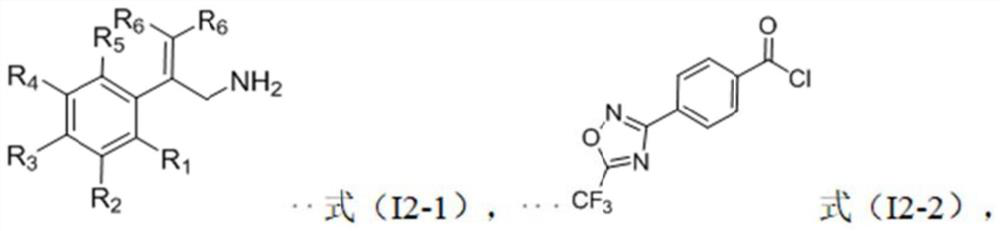Trifluoromethyl oxadiazole compound, preparation method and application thereof, and bactericide
A technology of trifluoromethyl oxadiazoles and compounds, which is applied in the field of pesticides, can solve problems such as resistance problems, and achieve excellent control effects and good crop safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0076] Preparation Example 1: Preparation of Compound 86
[0077] Step 1): Take triphenylmethyl phosphine bromide (60mmol, 1.5eq.) in a 250mL eggplant-shaped flask, add ultra-dry 100mL tetrahydrofuran, and then add potassium tert-butoxide (60mmol, 1.5eq. .), the solution turned yellow, continue to stir for 30min and then add o-chloroacetophenone (40mmol, 1eq.), after adding, move to room temperature to continue the reaction for 2h, after TLC monitoring the reaction is complete, add water to quench the reaction, ethyl acetate Extraction, washing 3 times with water and saturated brine each, mixing the organic phase with silica gel, using petroleum ether as eluent, and column chromatography to obtain the phenene intermediate.
[0078] Step 2): Take the alkene intermediate (30mmol, 1eq.) obtained in the previous step into a 250mL eggplant flask, add 60mL chloroform, then add NBS (33mmol, 1.1eq.), and finally add p-toluenesulfonic acid (9mmol). , 0.3eq.), reflux reaction for 1.5h,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com