Ursodeoxycholic acid pharmaceutical composition and preparation method and pharmaceutical preparation thereof
A technology of ursodeoxycholic acid and composition, which is applied in the fields of ursodeoxycholic acid pharmaceutical composition and its preparation and pharmaceutical preparation, can solve the problems of limited micronization solubilization effect, inability to dissolve rapidly the drug and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
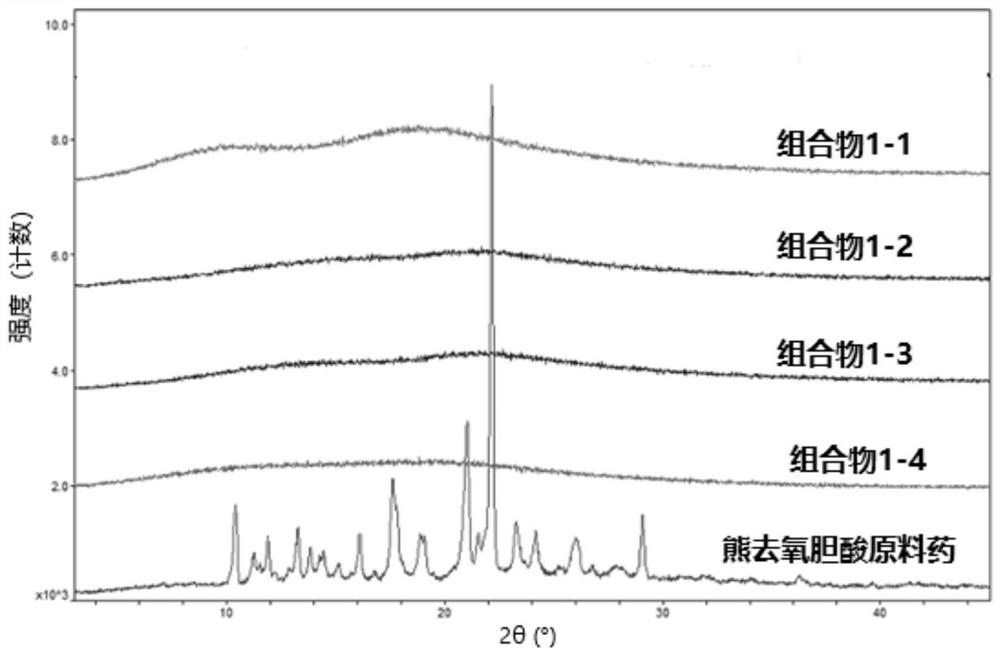
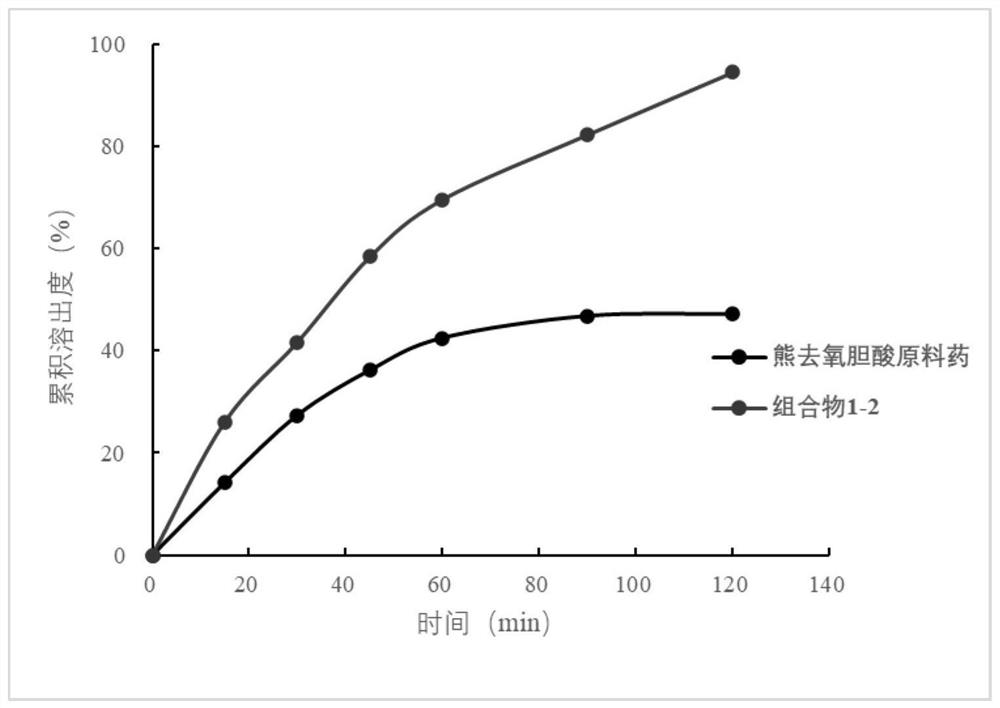
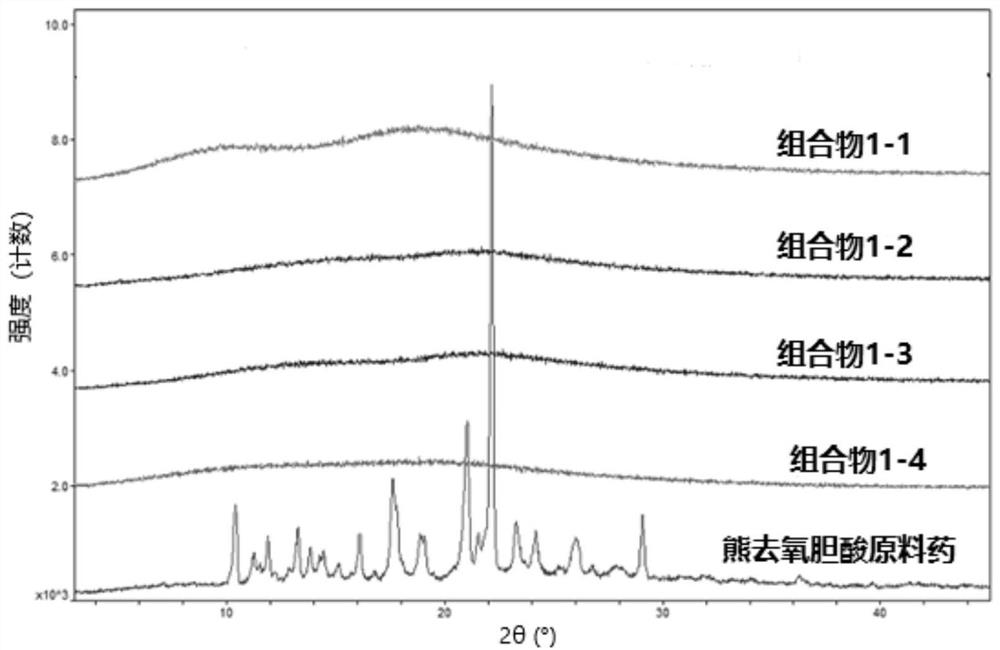
Image
Examples
preparation example Construction
[0082]There is no particular limitation on the cooling method described in the preparation method of the present invention, which may include air cooling, water cooling, mechanical cooling and the like.
[0083] There is no particular limitation on the type of extruder suitable for use in the present invention, including but not limited to single-screw or twin-screw type hot melt extruders. In one embodiment of the present invention, the extruder used to prepare the pharmaceutical composition of the present invention is a twin-screw type extruder. In this case, there is no particular limitation on the type of screw rotation, which includes, but is not limited to, co-rotating twin screws, counter-rotating twin screws, and biconical screw rotation patterns.
[0084] In a preferred embodiment of the present invention, the extruder used to prepare the pharmaceutical composition of the present invention is preferably a co-rotating twin-screw extruder. The temperature set by the ho...
Embodiment 1
[0104] Example 1 The preparation method and evaluation of ursodeoxycholic acid-polyvinylpyrrolidone-vinyl acetate copolymer pharmaceutical composition
[0105] 1. Preparation
[0106] Ursodeoxycholic acid- The composition and dosage of each component of the VA64 pharmaceutical composition are shown in Table 1-1.
[0107] Preparation method: ursodeoxycholic acid or its pharmaceutically acceptable salt and carrier materials are prepared according to the dosage shown in Table 1-1 VA64 or stoichiometric carrier material VA64 and solubilizer are mixed directly or uniformly in a mixer and then fed into the hopper of a co-rotating twin-screw extruder (Omicron 12 from India Steer Company), and the temperature of the co-rotating twin-screw extruder is controlled at about 120°C - Extrusion at about 190°C with a screw speed of about 100 to about 400 rpm. The obtained extrudate is cooled, crushed and sieved to obtain ursodeoxycholic acid- VA64 pharmaceutical composition.
[0108...
Embodiment 2
[0129] Example 2 Preparation method and evaluation of ursodeoxycholic acid-polyethylene glycol / vinyl caprolactam / vinyl acetate copolymer pharmaceutical composition
[0130] 1. Preparation
[0131] Ursodeoxycholic acid- The combination of the pharmaceutical composition and the dosage of each component are shown in Table 2-1.
[0132] Table 2-1 Ursodeoxycholic acid- The composition of pharmaceutical composition and each component consumption (weight g)
[0133]
[0134] Preparation method: mix ursodeoxycholic acid and carrier materials according to the dosage shown in Table 2-1 or stoichiometric carrier material Mix with Span 20 directly or evenly in a mixer and then feed into the hopper of a co-rotating twin-screw extruder (Omicron 12 from India Steer Company), and control the temperature of the co-rotating twin-screw extruder at about 120°C - Extrusion at about 190°C with a screw speed of about 100 to about 400 rpm. The obtained extrudate is cooled, crushed and si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com