Anodic oxidation of 5-aminouracil
An aminopyrimidine, anodizing technology, used in organic chemistry, medical preparations containing active ingredients, pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
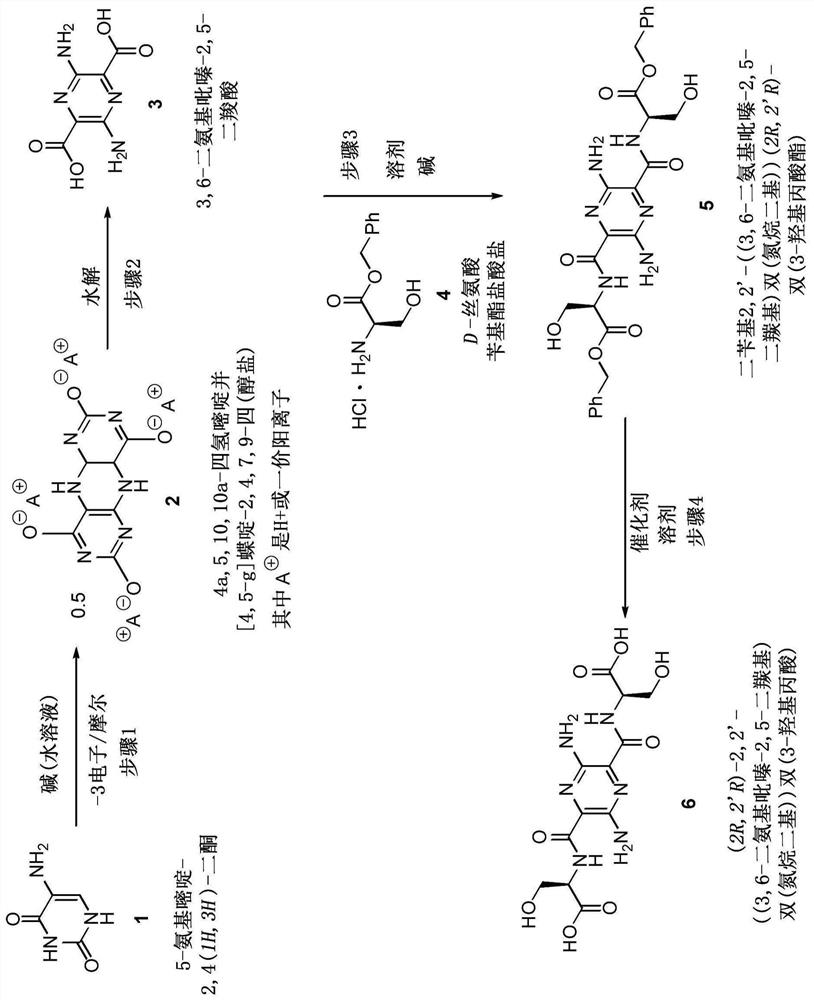
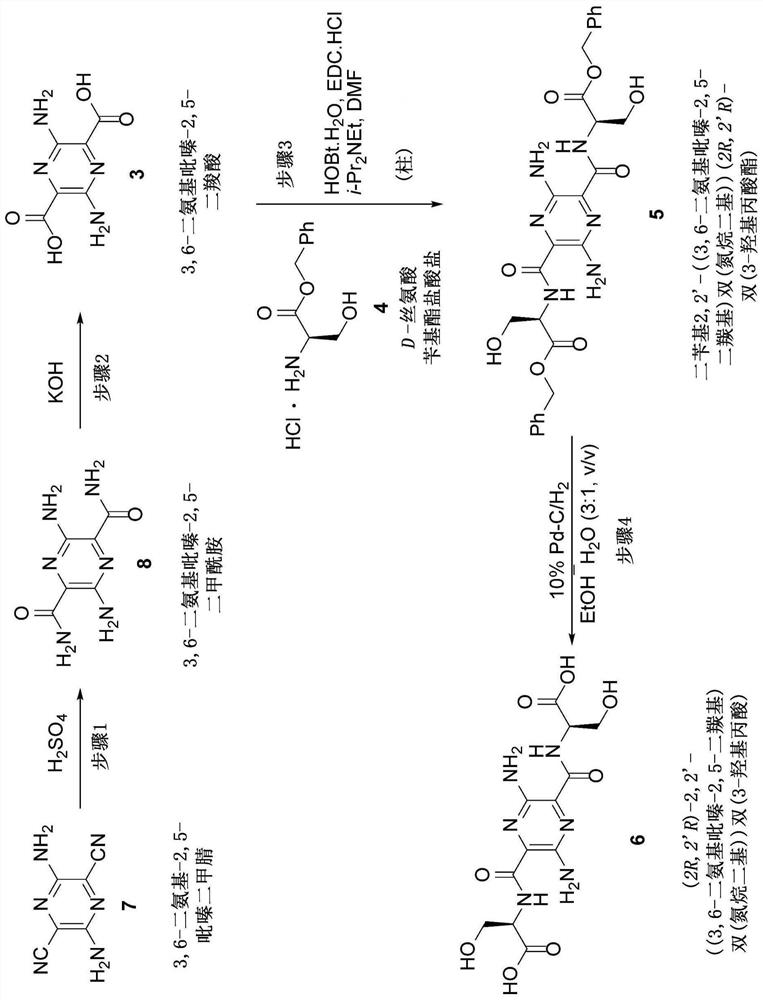
[0041] General methods for amide formation by coupling of a compound having an amine substituent (eg, compound 4) and a compound having a carboxyl substituent (eg, compound 3) are known to those skilled in the art. Specifically, US 2018 / 0010881 (incorporated herein by reference in its entirety) discloses the formation of compounds, compound 3,6-diamino-N, as shown below 2 ,N 5 -Bis(D-serine)-pyrazine-2,5-dicarboxamide. In step 1, 3,6-diamino-N was formed as shown below 2 ,N 5 -Bis(O-benzyl-(D)-serine methyl ester)-pyrazine-2,5-dicarboxamide. Sodium 3,6-diaminopyrazine-2,5-dicarboxylate (300 mg, 1.24 mmol), (D)-Ser(OBn)-OMe-HCl salt (647 mg, 2.64 mmol), A mixture of HOBt-H2O (570 mg, 3.72 mmol) and EDC-HCl (690 mg, 3.60 mmol) in DMF (25 mL). The resulting mixture was stirred for 16 h and concentrated. The mixture was concentrated to dryness and the residue was partitioned with EtOAc and water. The layers were separated with saturated NaHCO 3 The EtOAc solution was washe...
Embodiment 1
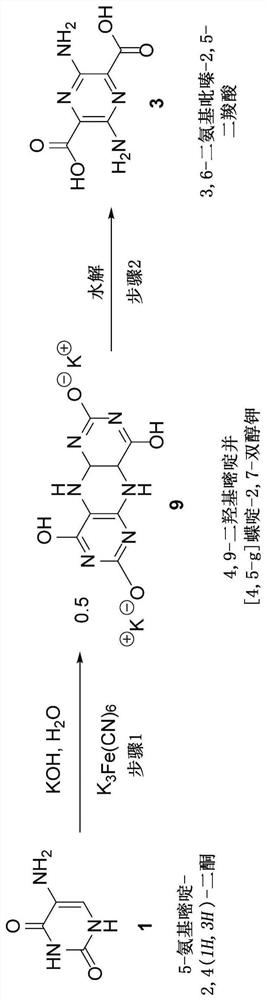
[0104] Potassium 4,9-dihydroxypyrimido[4,5-g]pteridine-2,7-diol was prepared according to the following reaction scheme:
[0105]
[0106] In the first (anode) chamber, a platinum-coated titanium expansion screen was fitted to the inner wall of a Bowers beaker (tall, 400 mL), and a graphite felt anode was placed uniformly on it and lined the inner wall. A stainless steel cathode formed from a 0.25 inch tube was placed in a pvc tube approximately 3 / 4 inch (1.9 cm) outside diameter and approximately 6 inches (15.24 cm) long sealed with clear room temperature vulcanized ("RTV") silicone at one end. in a separate (cathode) chamber formed. The tube was perforated by drilling holes at regular intervals of 1 / 4 inch (0.64 cm) to a distance of approximately 4 inches (10.2 cm) from the bottom of the RTV plug. With 0.15mm (0.006 inch) thick Nafion N324 film, Teflon TM Fabric reinforced (Rf[OCF 2 CF (CF 3 ) 2 ] n OCF 2 CF 2 SO 3 H) Wrap the pvc support tube with the rough side...
Embodiment 2
[0110] 3,6-Diaminopyrazine-2,5-dicarboxylic acid was prepared from potassium 4,9-dihydroxypyrimido[4,5-g]pteridine-2,7-diol according to the following reaction scheme:
[0111]
[0112]In each of the two Teflon reaction vessels place 0.5 g of potassium 4,9-dihydroxypyrimido[4,5-g]pteridine-2,7-diol and 0.3 to 10 mL of deionized water A solution of 0.4 g of sodium hydroxide. The vessel was set in a microwave reactor and allowed to react at 170°C and about 100 psig pressure for 1 hour. The vessel was cooled to about 50°C and the contents were filtered to remove a small amount of solid residue. The bright yellow filtrate was transferred to a 250 mL round bottom flask equipped with a large magnetic stir bar. The pH was adjusted to about 3 with stirring with concentrated HCl, resulting in the formation of a large red precipitate. A few drops of acid were added and the solid was collected by filtration on fritted glass, rinsed with cold 1N HCl (1 x 10 mL), acetonitrile (2 x 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com