Oligonucleotide-based treatment for ulcerative colitis
An oligonucleotide and dinucleotide technology, used in the field of comparably used in the treatment of inflammatory bowel diseases such as active ulcerative colitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Example 1 - Clinical Trial Study
[0178] The randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of topical cobitomod in patients with moderately to severely active ulcerative colitis according to established methods.
[0179] Methods: Men and women were selected for trials according to standard inclusion criteria in the field, including the following:
[0180] 1. Male or female ≥18 years old
[0181] 2. Diagnosed with UC, the shortest time since the diagnosis is ≥3 months
[0182] 3. Moderately to severely active left-sided UC (disease should extend 15 cm or more above the anal verge and not extend beyond the splenic flexure), as assessed by a modified Mayo score of 6 to 12 (excluding fragility of the endoscopic sub-score) Level 1) determination in which an endoscopic sub-score ≥2 as assessed by centralized interpretation of endoscopy performed at Screening Visit 1b (Day 7 to Day 10 Screening Visits) and no other individual sub-score...
reference example 1
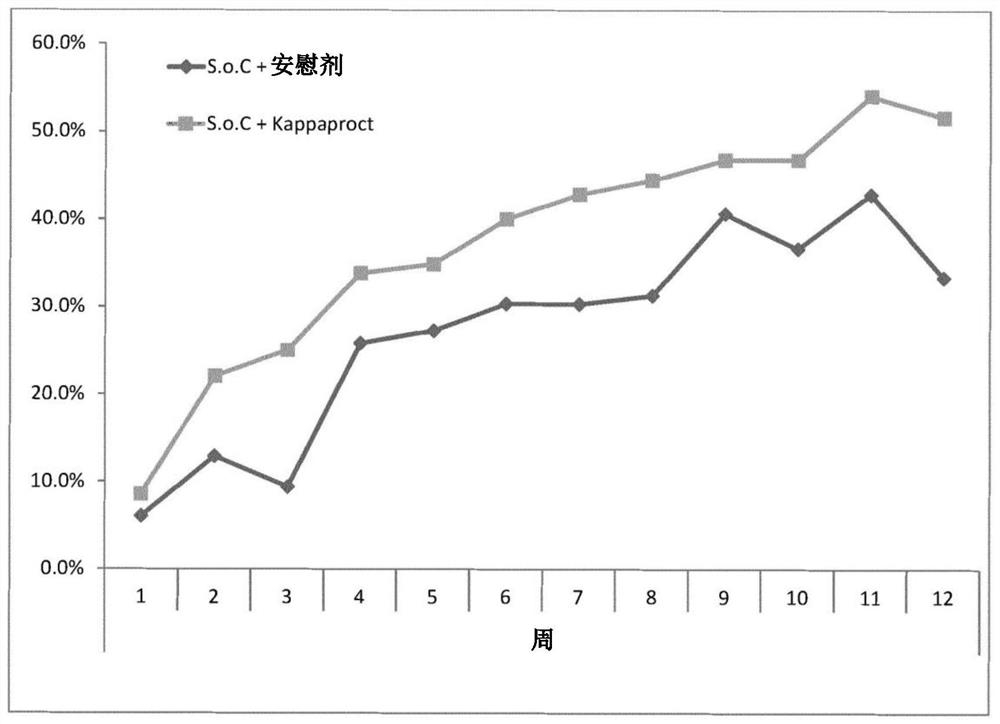
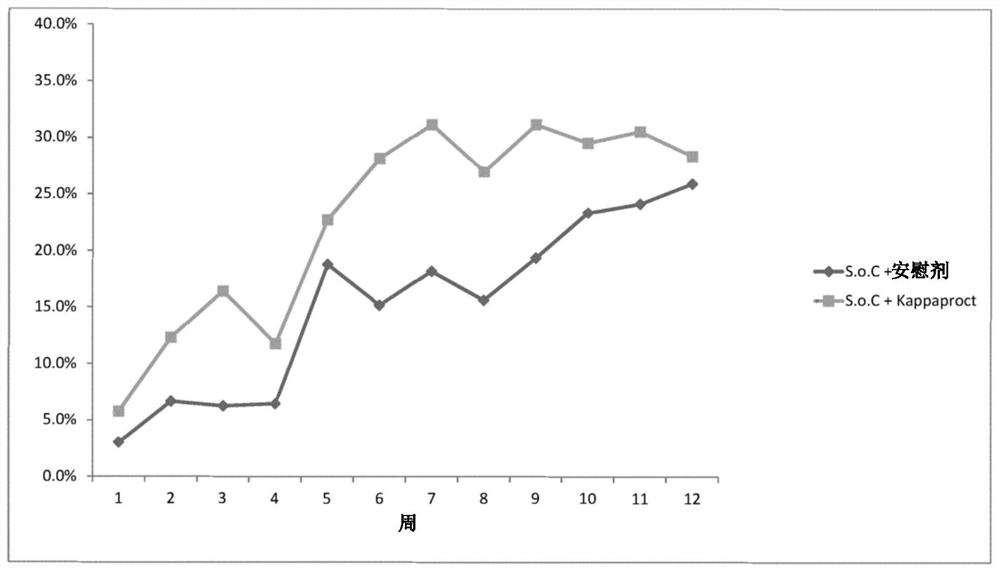
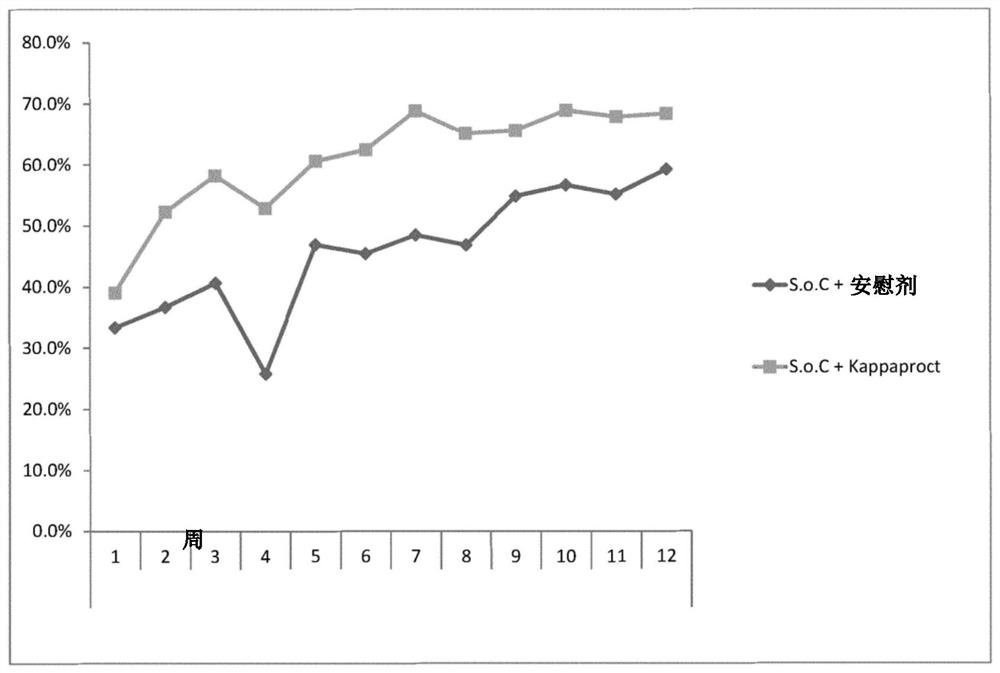
[0246] Reference Example 1 – Clinical Trial Results Showing Optimal Dosing Frequency
[0247] In a randomized, double-blind, placebo-controlled trial, 131 patients with moderately to severely active ulcerative colitis were randomized to receive two single doses of cobitolimod / Kappaproct (30 mg) or placebo at baseline and 4 Weekly topical application during lower GI endoscopy.
[0248] Patients in the treatment and placebo groups used electronic diaries to monitor weekly maximum blood volume in their stools (none, small, or large), weekly bowel frequency (eg, <18, 18-35, 36-60, or 61+), and weekly bowel movements. Daily bowel frequency (eg, <1, 1-1.99, 2-2.99, 3-3.99, 4-4.99, 5-5.99, 6-6.99, 7-7.99, or 8+) for 12 weeks.
[0249] Results were collated and the treatment delta calculated for the treatment group relative to the placebo group. As can be seen from these results, there is a particularly high therapeutic differential 3 weeks after the initial administration.
[0250...
Embodiment 2
[0266] Example 2 - Dextran Sodium Sulfate (DSS) Induced Colitis Mouse Model
[0267] Materials and methods
[0268] Mice: Balb / c mice were purchased from Charles River Laboratories, Research Models and Services (Sulzfeld, Germany). Eight-week-old female Balb / c mice were used for the experiments and were housed in individually ventilated cages in accordance with the Animal Welfare Act. Water and food were available ad libitum.
[0269] DSS-induced colitis: 3% (w / v) Dextran Sulfate Sodium (DSS) (MP Biomedicals, Illkirch, France) was administered to the drinking water of 8 week old female Balb / c mice for 10 days. An additional control group of three mice that were completely untreated was also part of the experimental setup. Food intake and body weight were monitored on days 0, 2, 4, 6, 7, 8 and 10.
[0270] Rectal administration of cobitolmod: 40 μg, 84 μg, 1000 μg or 1560 μg of cobitolmod were administered rectally twice per mouse (on days 4 and 8). Each concentration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com