Two-dimensional or quasi-two-dimensional polymer film synthesis method, two-dimensional or quasi-two-dimensional polymer film and application thereof
A polymer film, monomer polymerization technology, applied in the field of film production, electronic devices or catalysts, can solve the problems of small crystal domain size, reliable function limitation, unsatisfactory crystallinity of 2D polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
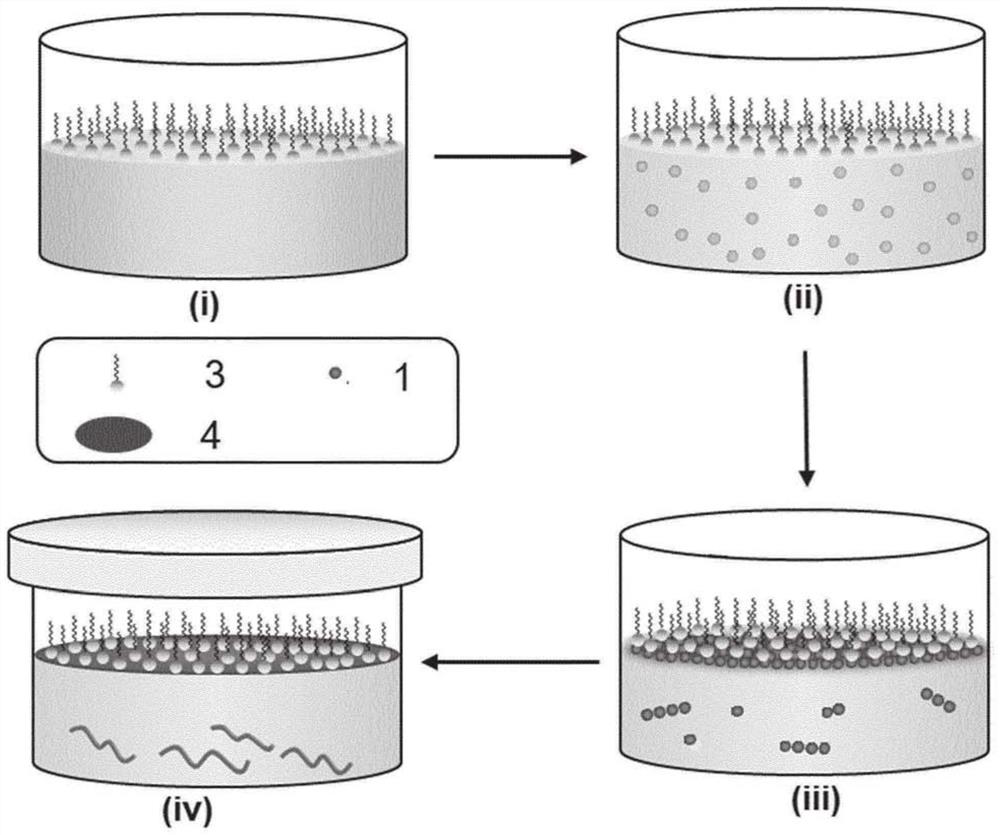
[0122] Formation of surfactant monolayers
[0123] Dissolve 20 μL of sodium oleyl sulfate (SOS) (1 mg ml in chloroform) -1 ) on the surface of 100 ml of Milli-Q water in a 200 ml beaker. The average molecular area (mma) is It can be calculated by the following formula:
[0124]
[0125] In the formula, Ae=25.12cm 2 , is the effective area; M w =M w,sos =370.5g mol -1 , is the molecular weight of SOS; Na=6.02×10 23 mol -1 is Avogadro's constant; m=m sos = 20 μg, which is the mass of the surfactant. The mma allows SOS molecules to form monolayers such as Figure 3A confirmed by the π-A isotherm in .
[0126] Similar to SOS, 15 μl stearic acid (SA, 1 mg ml in chloroform -1 ) was distributed at the interface between air and 100 ml of Milli-Q water. mma is allows SA molecules to form monolayers such as Figure 3B confirmed by the π-A isotherm in .
[0127] Horizontal synthesis of 2D polyimide (2DPI) on water
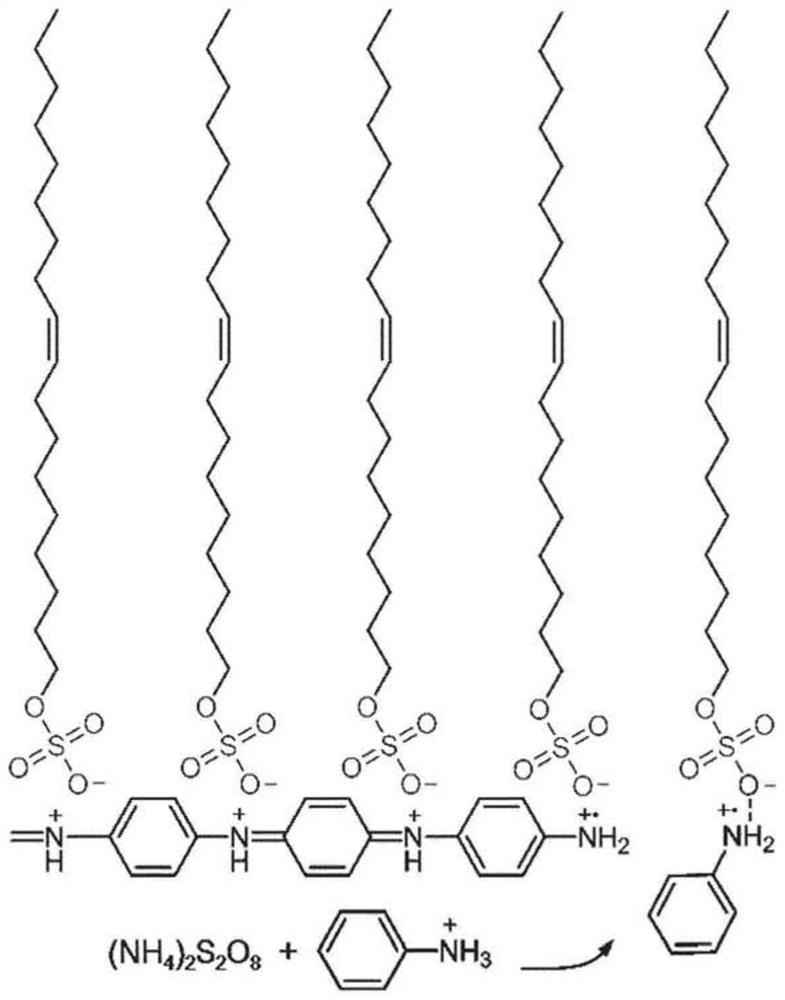
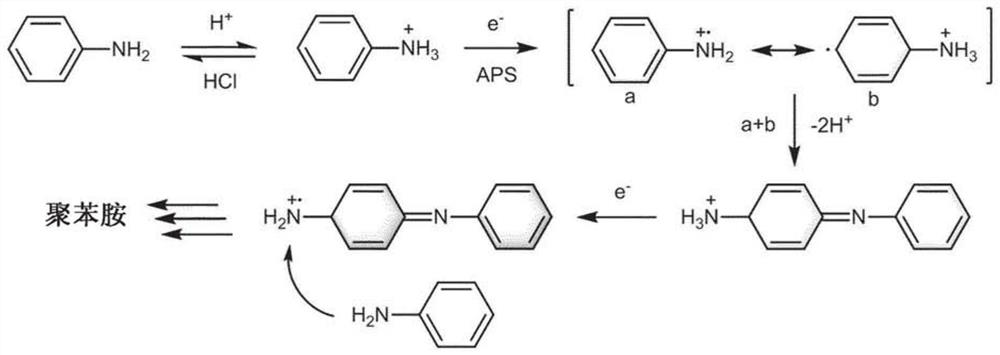
[0128] To synthesize 2DPI, SOS monolayers wer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com