Transduction plasmid, lentiviral vector system containing same and application of transduction plasmid
A plasmid and nucleotide sequence technology, applied in the field of biomedicine, can solve the problems of short continuous expression time of TCR and low lentiviral transduction efficiency, and achieve the effect of strong continuous expression ability and high transduction efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Construction of TCR lentiviral transduction plasmid vectors of different promoters
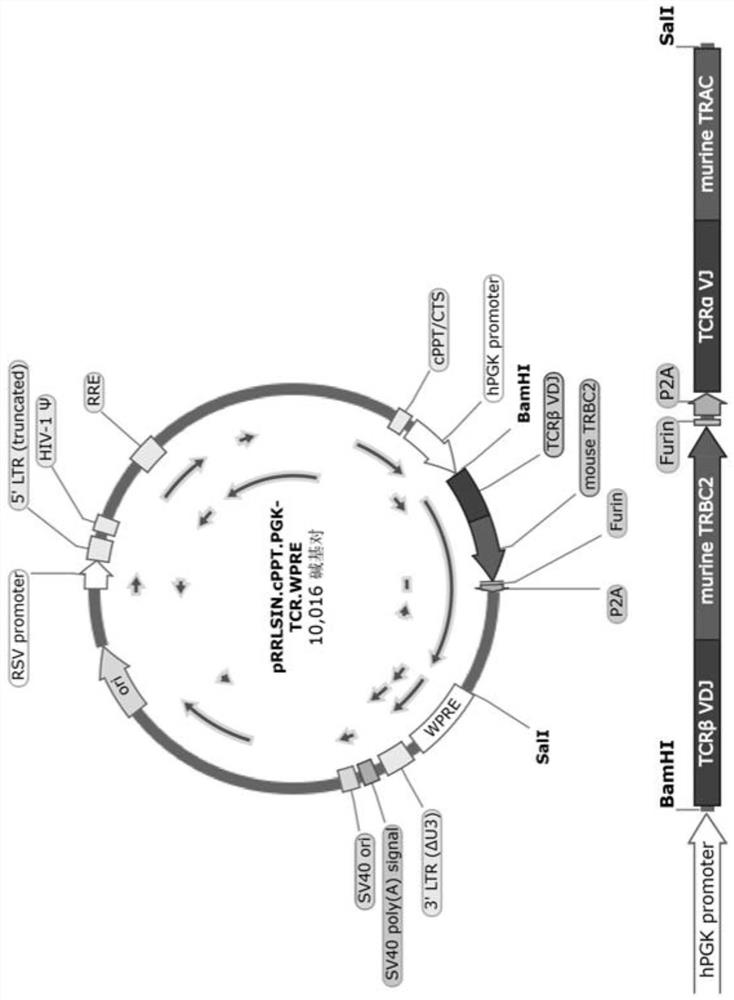
[0041] The TCR sequence used in the present invention is a MART-1 specific TCR, and the TCR specifically recognizes the melanoma tumor-associated antigen MART-1. The MART-1 specific TCRα variable region (SEQ ID No: 9) and TCRβ variable region (SEQ ID No: 10) sequences were compared with the mouse TCRα constant region (SEQ ID No: 7) and TCRβ2 constant region, respectively (SEQ ID No: 8) was fused to obtain human-mouse chimeric TCRα and TCRβ chains, and the two were combined with the 2A sequence (P2A, porcine teschovirus-1 2A, nucleotide sequence of Porcine teschovirus) of the internal self-cleaving porcine teschovirus (Porcine teschovirus). The sequence is shown in SEQ ID No: 4), and a paired alkaline amino acid protease cleavage site (furin cleavage site, SEQ ID No: 5) and an amino acid spacer (SEQ ID No: 6) are introduced before the 2A sequence. ),like figure 1 shown. I...
Embodiment 2
[0062] Example 2. Packaging and collection of lentivirus
[0063] Lentiviruses were packaged with the three lentiviral transduction plasmids constructed in 1. Lentiviral packaging was carried out using 293t cell line (purchased from ATCC), which was passaged one day before transfection, and transfected the next day when the confluence reached about 70%. Firstly, the medium was changed, and then three plasmids: 5T-4PGK transduction plasmid, PsPAX2 and pMD2.G (purchased from Addgene) were added to 500 μL of Opti-MEM solution in a mass ratio of 4:3:2. Polyethylenimine (PEI) with 3 times the mass of the plasmid was added to 500 μL of Opti-MEM solution, the two solutions were thoroughly mixed, and incubated at room temperature for 20 min. Gently add to the medium and shake side to side.
[0064] The first batch of virus was collected in 48 hours, and the supernatant of the medium was taken at 2000 RPM. After centrifugation for 10 minutes, it was passed through a 0.45 μM filter m...
Embodiment 3
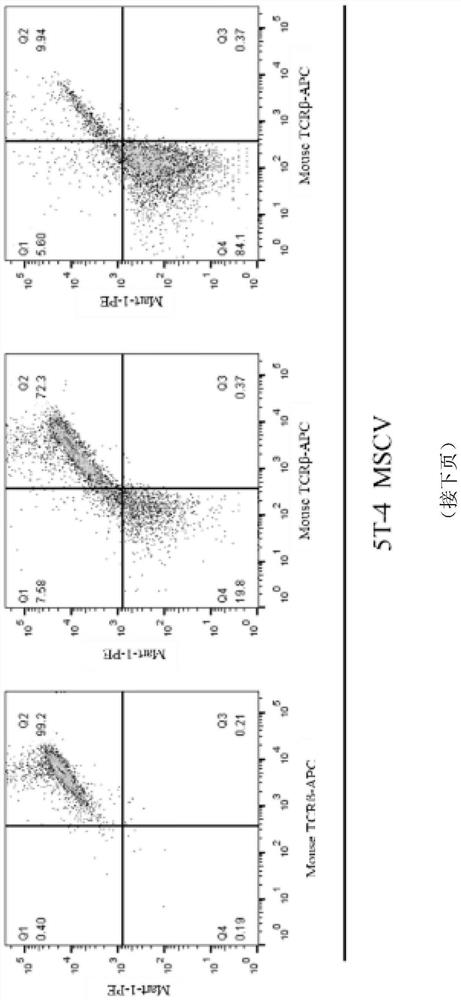
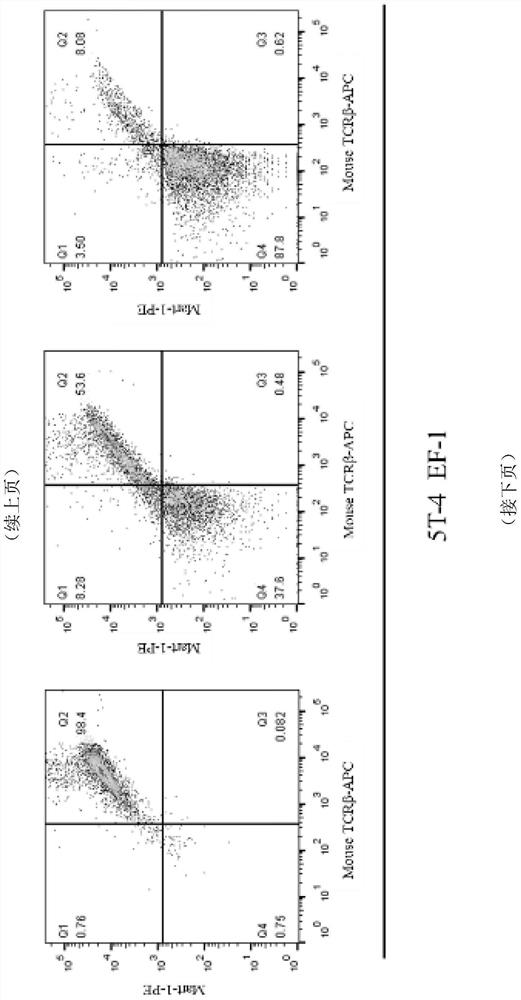
[0066] Example 3. Detection of TCR positive rate of Jurkat cell line infected by lentiviral vectors with different promoters
[0067] In order to compare the expression efficiency and persistence of the TCR in the three lentivirus-infected cells prepared in Example 2, the virus was infected with serial dilutions of Jurkat cell line (purchased from ATCC).
[0068] The specific experimental process is as follows:
[0069] Before infection, the cell concentration was adjusted to count 1 x 10 6 pcs / ml. A 96-well plate was plated, 100 μL per well, and polybrene, a pro-infection reagent, was added to the cell resuspension at a final concentration of 6 μg / ml. The amount of lentivirus added was set to three gradients, namely 0.1, 1, and 10 μL three gradients. After adding lentivirus, pipette mixed evenly, centrifuge at 800g for 30 minutes to make the virus and cells fully contact, and then use a pipette to mix evenly and put it in. , 37℃, 5%CO 2 Cultivated in an incubator. Sinc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com