Biomarker for diagnosis of preeclampsia of pregnancy, application and kit
A diagnostic kit and biomarker technology, applied in the field of biomedicine, can solve problems such as non-compliance with diagnostic standards, and achieve the effects of strong specificity and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
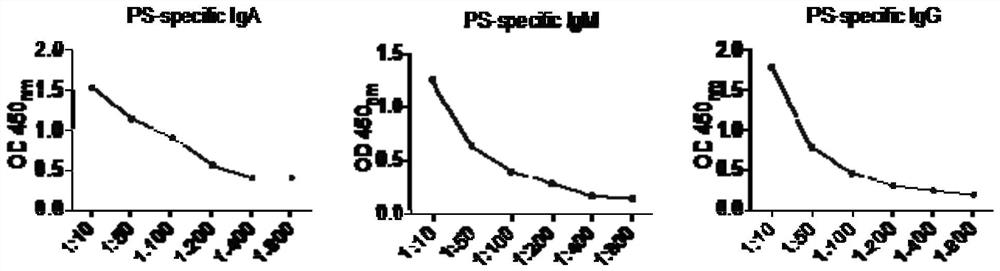
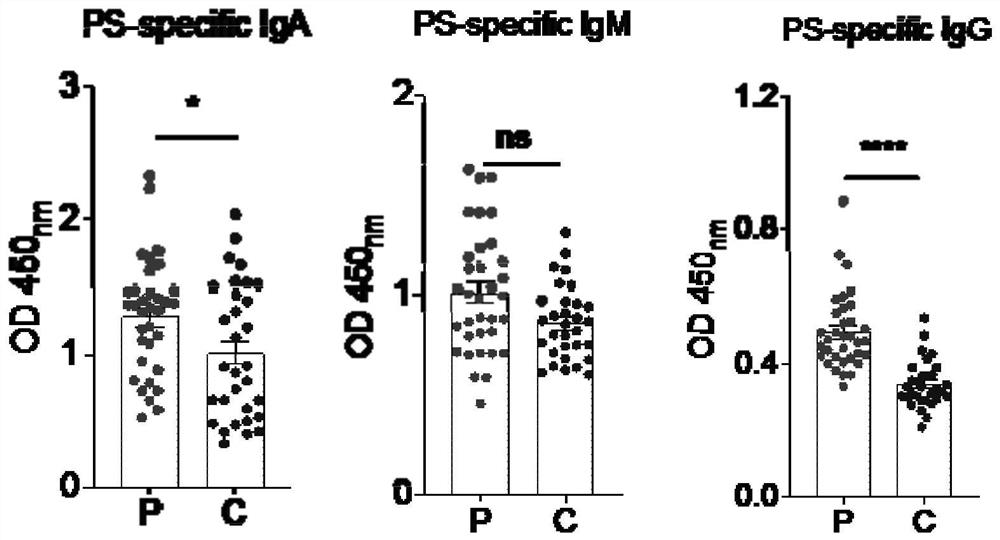
[0026] This program detects the concentrations of αPS antibody and its subtypes IgA, IgG, and IgM in the plasma of pregnant women with preeclampsia (12 weeks of gestation) and healthy pregnant women (12 weeks of gestation), that is, the concentrations of αPSIgA, αPSIgG, and αPSIgM. Correlation analysis between autoantibody levels and clinical indicators, including the following experimental steps:
[0027] 1. Collection of serum samples: blood samples were collected with vacuum coagulant blood collection tubes, and fresh whole blood ( 12 weeks of pregnancy), mix up and down, let stand at room temperature for 30 minutes, centrifuge at 3000 rpm for 5 minutes, draw the upper serum for detection, obtain plasma samples, select one of the plasma samples of pregnant women with preeclampsia and PBS at 1:10, 1:50, The ratios of 1:100, 1:200, 1:400 and 1:800 were diluted and stored for later use. The remaining samples were diluted with PBS at the ratio of 1:100 and stored for later use....
Embodiment 2
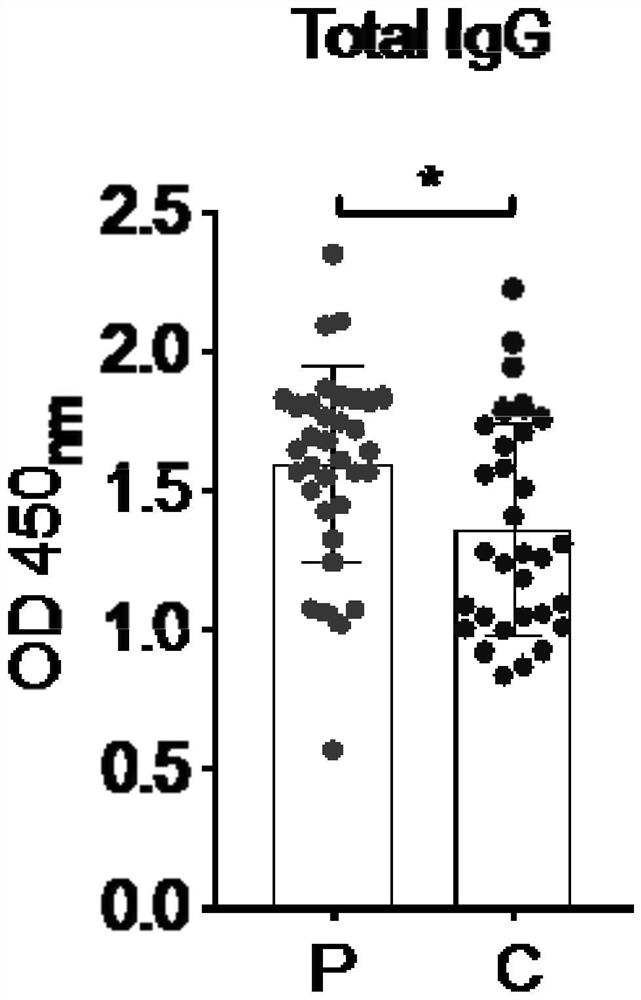
[0037] This protocol detects the concentration of total IgG in the plasma of pregnant women with preeclampsia (12 weeks of gestation) and healthy pregnant women in the first trimester (12 weeks of gestation), including the following steps:
[0038] 1. Collection of serum samples: blood samples were collected with vacuum coagulant blood collection tubes, and fresh whole blood ( 12 weeks of pregnancy), mixed up and down, left at room temperature for 30 minutes, centrifuged at 3000 rpm for 5 minutes, sucked the upper serum for detection, and obtained plasma samples. The samples were diluted 1:100 with PBS and stored for future use.
[0039] 2. Antigen coating: 100ug of Purified anti-human IgG Fc Recombinant Antibody (BioLegend, California, USA, 366902) was added to PBS and diluted to a final concentration of 5ng / ml; 60ul of PS antigen was added to each well of the ELISA plate; sealed 96 The well plate was placed in a 4°C refrigerator for overnight incubation; the 96-well plate w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com