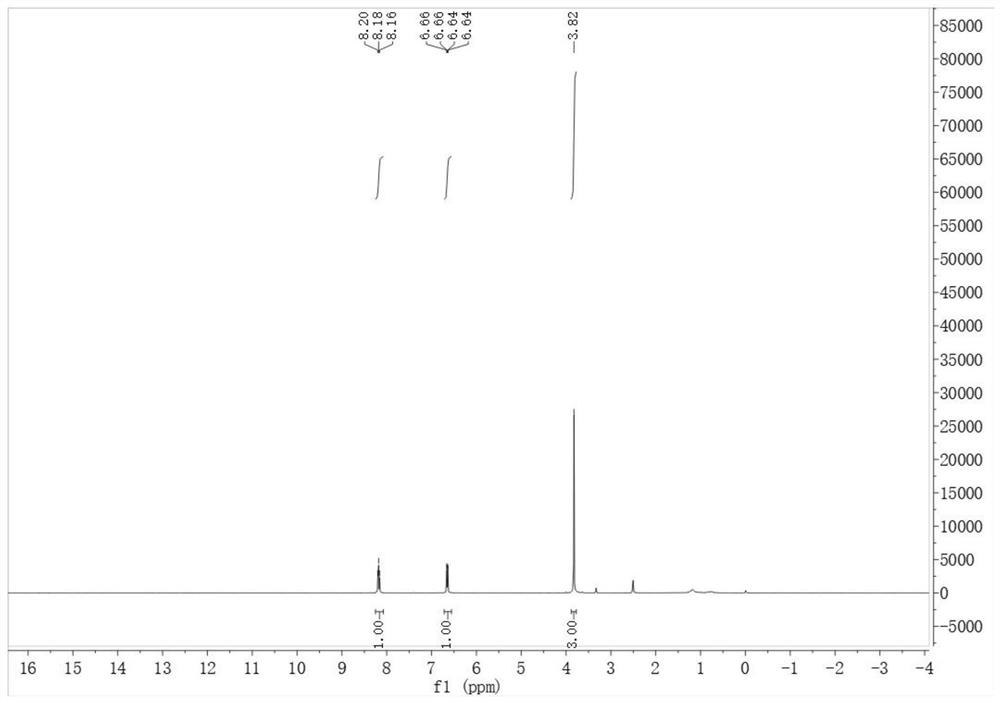
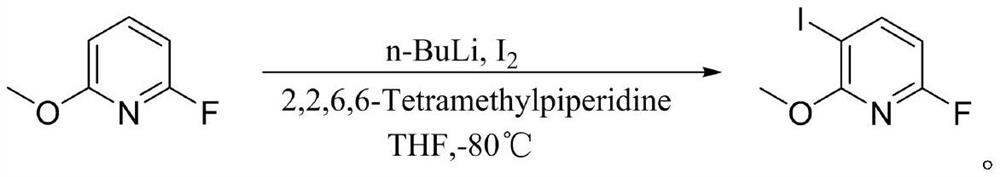Preparation method of 6-fluoro-3-iodine-2-methoxypyridine
A technology of methoxypyridine and n-butyllithium, applied in the direction of organic chemistry and the like, can solve the problems of time-consuming and labor-intensive purification methods, high synthesis cost, poor selectivity, etc., to simplify post-processing operations and purification methods, and to solve synthetic problems Cost issue, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of preparation method of 6-fluoro-3-iodo-2-methoxypyridine, its concrete steps are:
[0025] Vacuum the 3L three-neck glass flask to -0.07Mpa with a circulating water vacuum pump, break the vacuum with nitrogen, and repeat the cycle three times; then vacuum the reaction flask to -0.06Mpa; then add 2, 2, 6, 6- The THF (290ml) solution of tetramethylpiperidine (144.5g, 1022.7mmol) was sucked into the reaction flask, and the reaction device was replaced by nitrogen three times; the reaction flask was cooled to -50°C with liquid nitrogen; Add n-butyllithium (409ml, 1022.7mmol) in n-hexane solution, control the temperature to -50°C during the dropwise addition; after dropping, stir at -50°C for 60 minutes; then continue to cool down to -85°C, add 2-fluoro- A solution of 6-methoxypyridine (100g, 786.7mmol) in THF (300ml) was slowly dropped into the reaction flask, pay attention to temperature control at -85°C, and stir the reaction at -85°C for 60 minutes; then add iod...
Embodiment 2
[0027] A kind of preparation method of 6-fluoro-3-iodo-2-methoxypyridine, its concrete steps are:
[0028] Vacuum the 3L three-neck glass flask to -0.06Mpa with a circulating water vacuum pump, break the vacuum with nitrogen, and repeat the cycle three times; then vacuum the reaction flask to -0.05Mpa; The THF (400ml) solution of tetramethylpiperidine (133.3g, 944.04mmol) was sucked into the reaction flask, and the reaction device was replaced by nitrogen for 3 times; the reaction flask was cooled to -40°C with liquid nitrogen; Add the n-hexane solution of n-butyllithium (409ml, 1022.7mmol), control the temperature to -40°C during the dropwise addition; after the dropwise addition, stir at -40°C for 70 minutes; then continue to cool down to -75°C, add 2-fluoro- A solution of 6-methoxypyridine (100g, 786.7mmol) in THF (400ml) was slowly dropped into the reaction flask, pay attention to temperature control at -75°C, and stir the reaction at -75°C for 65 minutes; then add iodine ...
Embodiment 3
[0030] A kind of preparation method of 6-fluoro-3-iodo-2-methoxypyridine, its concrete steps are:
[0031] Vacuum the 3L three-neck glass flask to -0.06Mpa with a circulating water vacuum pump, break the vacuum with nitrogen, and repeat the cycle three times; then vacuum the reaction flask to -0.06Mpa; The THF (350ml) solution of tetramethylpiperidine (155.6g, 1101.38mmol) was sucked into the reaction flask, and the reaction device was replaced with nitrogen three times; the reaction flask was cooled to -45°C with liquid nitrogen; Add n-butyllithium (409ml, 1022.7mmol) in n-hexane solution, and control the temperature to -45°C during the dropwise addition; after dropping, stir at -45°C for 80 minutes; then continue to cool down to -80°C, add 2-fluoro- A solution of 6-methoxypyridine (100g, 786.7mmol) in THF (350ml) was slowly dropped into the reaction flask, pay attention to temperature control at -80°C, and stir the reaction at -80°C for 70 minutes; then add iodine (279.8g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com