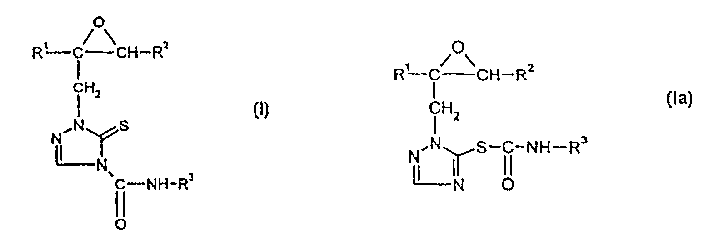
Oxyranyle-triazoline thiones and their use as microbicides
A technology of oxirane-based and triazolinethione, which is applied in biocides, animal repellents, plant growth regulators, etc., and can solve unsatisfactory problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241]
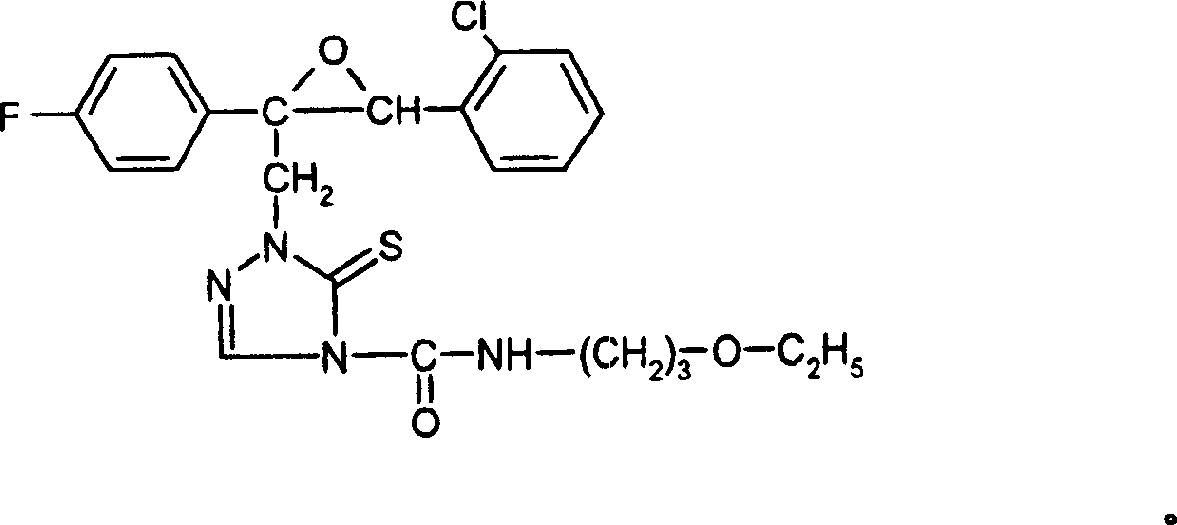
[0242] Under stirring at room temperature, 350 mg (0.97 mmol) of 3-(2-chloro-phenyl)-2-(4-fluoro-phenyl)-2-[(4,5-dihydro-5-thiono-1 , 2,4-triazol-1-yl)-methyl]-oxirane, a mixture of 0.1ml triethylamine and 5ml anhydrous tetrahydrofuran was added dropwise with 110mg (0.97mmol) isocyanide in 5ml anhydrous tetrahydrofuran Solution of acid 3-ethoxy-propyl ester. After the addition was complete, the reaction mixture was heated at 60°C for 1 hour, then concentrated under reduced pressure. The product that remains is chromatographed on silica gel using a cyclohexane / ethyl acetate = 4:1 mixture. Concentration of the eluate afforded 390 mg (82% of theory) of the substance of the formula given above.
[0243] 1 H-NMR spectrum (300MHz, CDCl 3 , TMS): δ=10.0(s, 1H); 84(s, 1H); 7.6-7.3(m, 6H); 7.0(t, 2H, J=8.7Hz); 5.1(d, 1H,
[0244] J=14.9Hz); 4.1(s, 1H); 3.7(d, 1H, J=14.9Hz); 3.5-3.4(m, 6H); 1.9-1.8(m,
[0245] 2H); 1.2(t, 3H, J=7.0Hz) ppm.
[0246] The compounds liste...
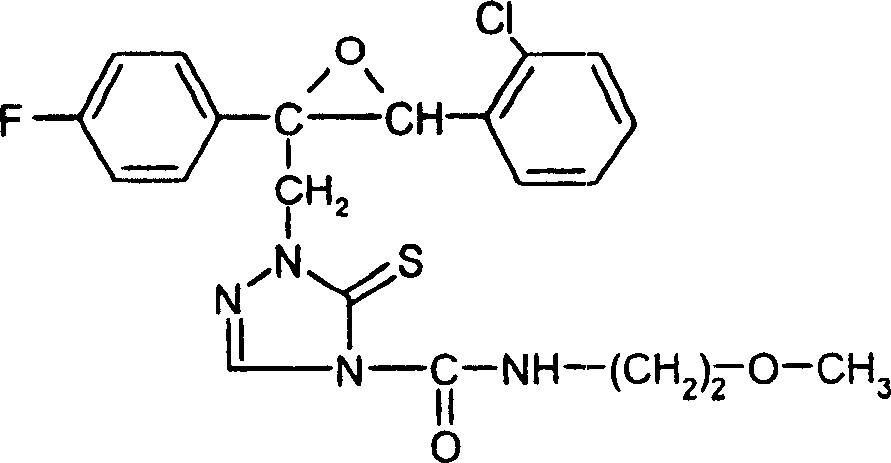
Embodiment 7
[0251]
[0252] 1 H-NMR spectrum (400MHz, CDCl 3 , TMS): δ=7.8(s, 1H); 7.3-7.1(m, 4H); 7.0-6.9(m, 4H); 6.7(s, 1H); 5.0(d, 1H); 4.7(d, 1H ); 4.5(s, 1H); 3.7(m, 1H); 1.0(d, 6H, J=6.6Hz)ppm.
Embodiment A
[0255] Powdery mildew (Erysiphe) test (barley) / protective
[0256] Solvent: 25 parts by weight of N,N-dimethylacetamide
[0257] Emulsifier: 0.6 parts by weight of alkyl aryl polyglycol ether
[0258] To prepare a suitable preparation of the active compound, 1 part by weight of the active compound is mixed with the stated amount of solvent and emulsifier, and the emulsifiable concentrate is diluted with water to the desired concentration.
[0259] To test for protective activity, young plants are sprayed with the preparation of active compound at the stated application rates.
[0260] After the spray coat has dried on, the plants are sprinkled with spores of wheat powdery mildew (Erysiphe graminis f. sp. hordei).
[0261] The plants are placed in a greenhouse at a temperature of about 20° C. and a relative air humidity of about 80% to promote the growth of mildew spots.
[0262] Evaluations were performed 7 days after inoculation. 0% indicates an efficacy equivalent to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com