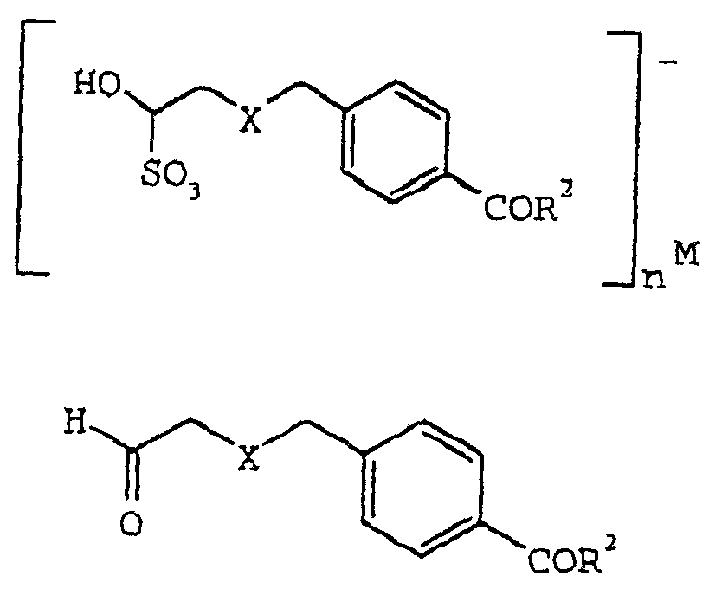
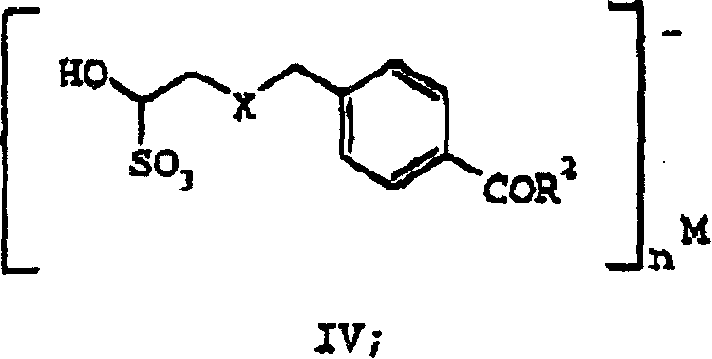
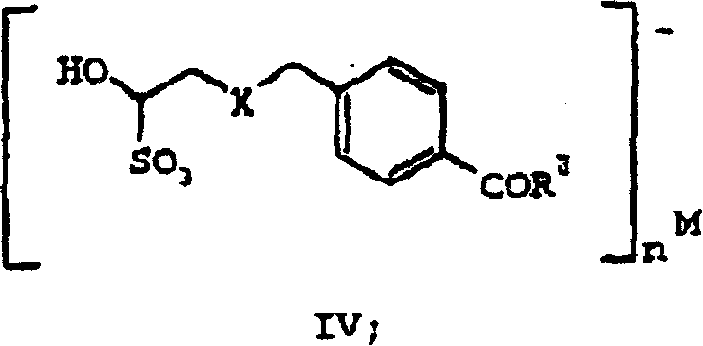
Processes and intermediates useful to make antifolates
A solvent and compound technology, applied in the field of synthetic organic chemistry, can solve problems such as difficulty in obtaining pure compound of general formula XIV, difficulty in separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] 4-(4-Methoxyphenyl)butyraldehyde
[0094] Deloxan used below THP Type 2 resin was pretreated by mixing with isopropanol (2.0 vol, 20 ml) and washing with ethyl acetate (4.0 vol, 40 ml). Filter the organic layer / resin slurry before subsequent use.
[0095] Methyl 4-bromobenzoate (60.0 g, 279.0 mmol), lithium acetate dihydrate (31.31 g, 306.9 mmol), lithium chloride (35.48 g, 837 mmol) and tetrabutylammonium chloride (41.22 g, 131.49 mmol) Add to dimethylformamide (698ml). The resulting solution was degassed by bubbling nitrogen. 3-Buten-1-ol (24.19g, 28.81ml, 334.81mmol) and palladium acetate (1.57g, 6.98mmol) were added and the reaction mixture was heated and stirred at 65°C for about 10 hours. Completion of the reaction was indicated by HPLC monitoring of consumption of starting material (less than 0.4% methyl 4-bromobenzoate remaining) (reverse phase, 60% acetonitrile: 2.5% acetic acid buffer). The reaction mixture was cooled to 25°-30°C, water (700ml) and ethy...
Embodiment 2
[0099] 1-Hydroxy-4-(4-methoxyphenyl)butanesulfonic acid sodium salt
[0100]The ethyl acetate extract of Example 1 was concentrated in vacuo at about 37°C to 3.6 vol (8.7 ml). Alcohol 3A (3 vol, 7.2 ml) and water (0.63 vol, 1.51 ml) were added, followed by sodium bisulfite (1.04 g, 10.03 mmol). The reaction mixture was stirred for about 8 hours. After 10 minutes, the sulfonic acid started to crystallize. through 1 H NMR analysis of the filtrate of the reaction mixture verified the completion of the reaction. The resulting white slurry was filtered to afford the title compound (2.78 g, 8.98 mmol) as a white crystalline solid in about 80% yield. The filter cake was washed with ethanol (1.8 vol) and dried under vacuum at 40°C. Isomer impurities were not detected by NMR.
[0101] 1 H-NMR: (d 6 -DMSO) δ7.86(d, J=8.27Hz, 2H), 7.32(d, J=8.27Hz, 2H), 5.33(d, J=2.3Hz, 1H), 3.84(m, 1H), 3.81( s, 3H), 2.63(m, 2H), 1.75(m, 1H), 1.73(m, 1H), 1.61(m, 1H), 1.48(m, 1H).
[0102] 13...
Embodiment 3
[0105] 1-Hydroxy-2-bromo-4-(4-methoxyphenyl)butyraldehyde
[0106] To a 50 ml round bottom flask equipped with magnetic stirring was added 4-(4-oxobutyl)methyl benzoate sodium bisulfite adduct (3.10 g, 10 mmol), acetonitrile (14 ml) and trimethylchlorosilane ( 3.6ml, 28mmol). Nitrogen was sparged for 5 minutes, then the mixture was heated at 60° C. for 1 hour under a nitrogen atmosphere. The reaction mixture was bright yellow at this point. The mixture was cooled to 5°C and bromine (0.5ml, 9.7mmol) was added. Within 1 minute, the bromine brown color disappeared. The solution was bright yellow and the visible solid was colorless. The mixture was removed from the cooling bath and stirred for an additional 2 hours. Water (14ml) and sodium bisulfite (0.14g) were added to quench the remaining bromine and the resulting mixture was stirred for 1 hour. The mixture was partitioned between dichloromethane (14ml) and a further 7ml of water. The organic phase was separated and strip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com