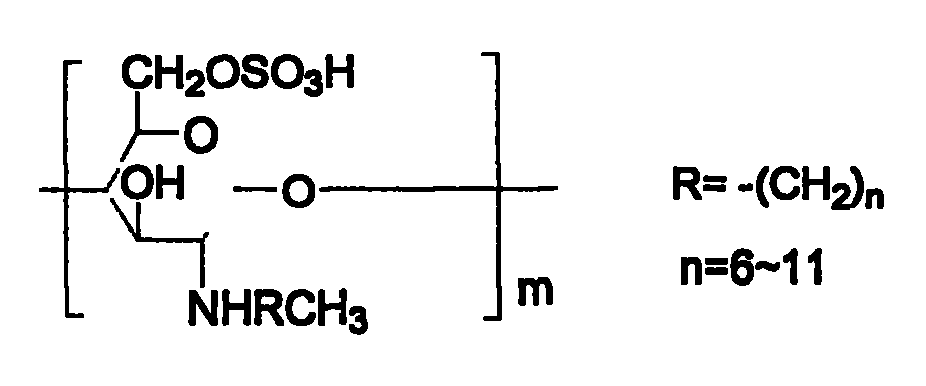
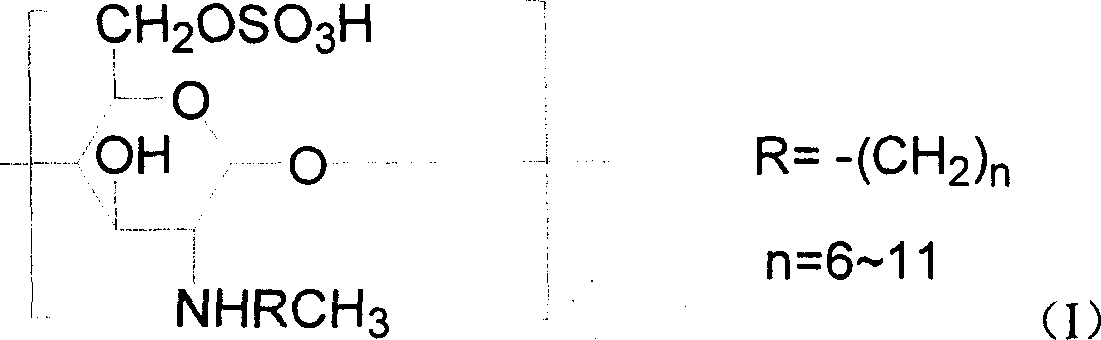
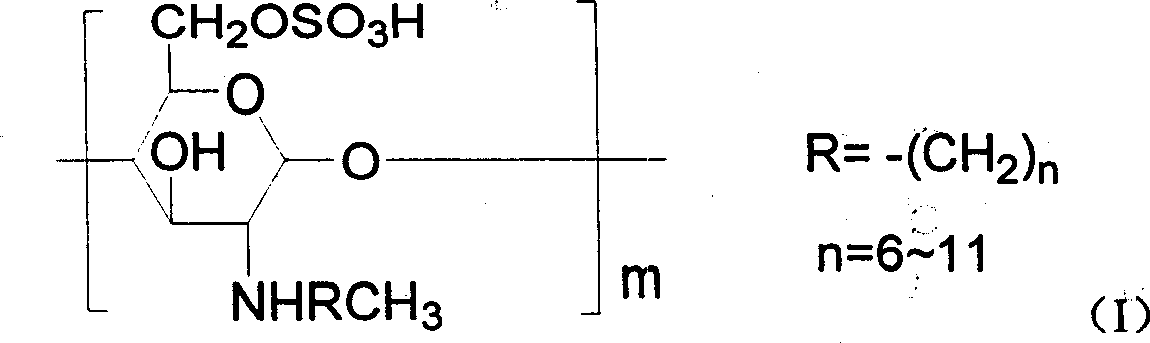Chitin derivative and its preparation and its uses in preparation of medicines
A technology of polysaccharides and drugs, applied in the field of application in the preparation of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] 1, the preparation of N-octyl chitosan (referring to literature: Baba Y, Hirakawa H.Chem Lett 1992,10,1905-1908)
[0079] Chitosan (1.0g) was suspended in 50ml of methanol and stirred at room temperature. Octanal (1.02g) was added. After continuing the reaction for 24 hours, 5ml of aqueous solution of KBH4 (0.5g) was added, and stirring was continued overnight. The reaction solution was washed with 2N hydrochloric acid solution. Neutralize, precipitate with methanol, filter, wash the filter residue three times with methanol and water, and dry at 60° C. in a vacuum oven overnight to obtain 1.0 g of light yellow powder N-octyl chitosan.
[0080] 2. Preparation of N-octyl-O-sulfonic acid chitosan (see literature: Hirano S, Tanaka Y, Hasegawa M, et al.Carbohy dr.Res.1985, 137, 205-215)
[0081] N-octyl chitosan (1.5g) was suspended in DMF (40ml) with mechanical stirring overnight, chlorosulfonic acid (20ml), at 0°C, N 2Under protection and stirring, it was slowly added dro...
Embodiment 2
[0087] 1. Preparation of N-decyl chitosan
[0088] Using decanal and chitosan to react, the preparation method is the same as that of N-octyl chitosan.
[0089] 2. Preparation of N-decyl-O-sulfonic chitosan
[0090] It is prepared by reacting N-decyl chitosan and chlorosulfonic acid, and the method is the same as that of N-octyl-O-sulfonic acid chitosan.
[0091] FT-IR: 2950, 2860, 1462, 1385, 800 cm-1 (long chain alkyl), 1260, 1221 1002 cm-1 (O=S=O).
[0092] 1H-NMR(D2O): δ(ppm): 3.0~3.3(m, 2H)(-NH-CH2-(CH2)8-CH3); 1.0~2.5(m, 8H)(-NH-CH2-(CH2 )8-CH3), 0.81(tri,3H)(-NH-CH2-(CH2)8-CH3).
[0093] 13C-NMR(D2O)δ(ppm): 13.5~13.7(-NH-CH2-(CH2)8-CH3); 22.3~34.2(-NH-CH 2 -(CH 2 ) 8 -CH 3 ); 48.0 (-NH-CH 2 -(CH 2 ) 8 -CH 3 );55.3(C 2 ), 62.1 (C 6 ), 65.9 (C 3 ), 75.7 (C 5 ), 77.1 (C 4 ), 97.5 (C 1 ).
[0094] Elemental analysis records that the degree of deacetylation is 91.5%, the degree of substitution of N-decyl group is 50%, and the degree of substitution of sul...
Embodiment 3
[0096] 1. Preparation of N-dodecyl-chitosan
[0097] React with lauryl aldehyde and chitosan, and the preparation method is the same as that of N-octyl chitosan.
[0098] 2. Preparation of N-dodecyl-O-sulfonic chitosan
[0099] It is prepared by reacting N-dodecyl chitosan with chlorosulfonic acid, and the method is the same as that of N-octyl-O-sulfonic acid chitosan.
[0100] FT-IR: 2952, 2865, 1462, 1385, 810cm -1 (long chain alkyl), 1265, 12211005cm -1 (O=S=O)
[0101] 1 H-NMR (D 2 O): δ (ppm): 2.6.0 ~ 3.4 (m, 2H) (-NH-CH 2 -(CH 2 ) 10 -CH 3 ); 0.8~3.3(m, 6H)(-NH-CH 2 -(CH 2 ) 10 -CH 3 ), 0.83(tri,3H)(-NH-CH 2 -(CH 2 ) 10 -CH 3 ).
[0102] 13 C-NMR (D 2 O)δ(ppm): 13.5~13.9(-NH-CH 2 -(CH 2 ) 10 -CH 3 ); 22.1~33.5(-NH-CH 2 -(CH 2 ) 10 -CH 3 ); 48.7(-NH-CH 2 -(CH 2 ) 10 -CH 3 );55(C 2 ), 61.8 (C 6 ), 67(C 3 ), 75.4 (C 5 ), 76.9 (C 4 ) and 97(C 1 ).
[0103] Elemental analysis records that the degree of deacetylation is 91.5%, the degree ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com