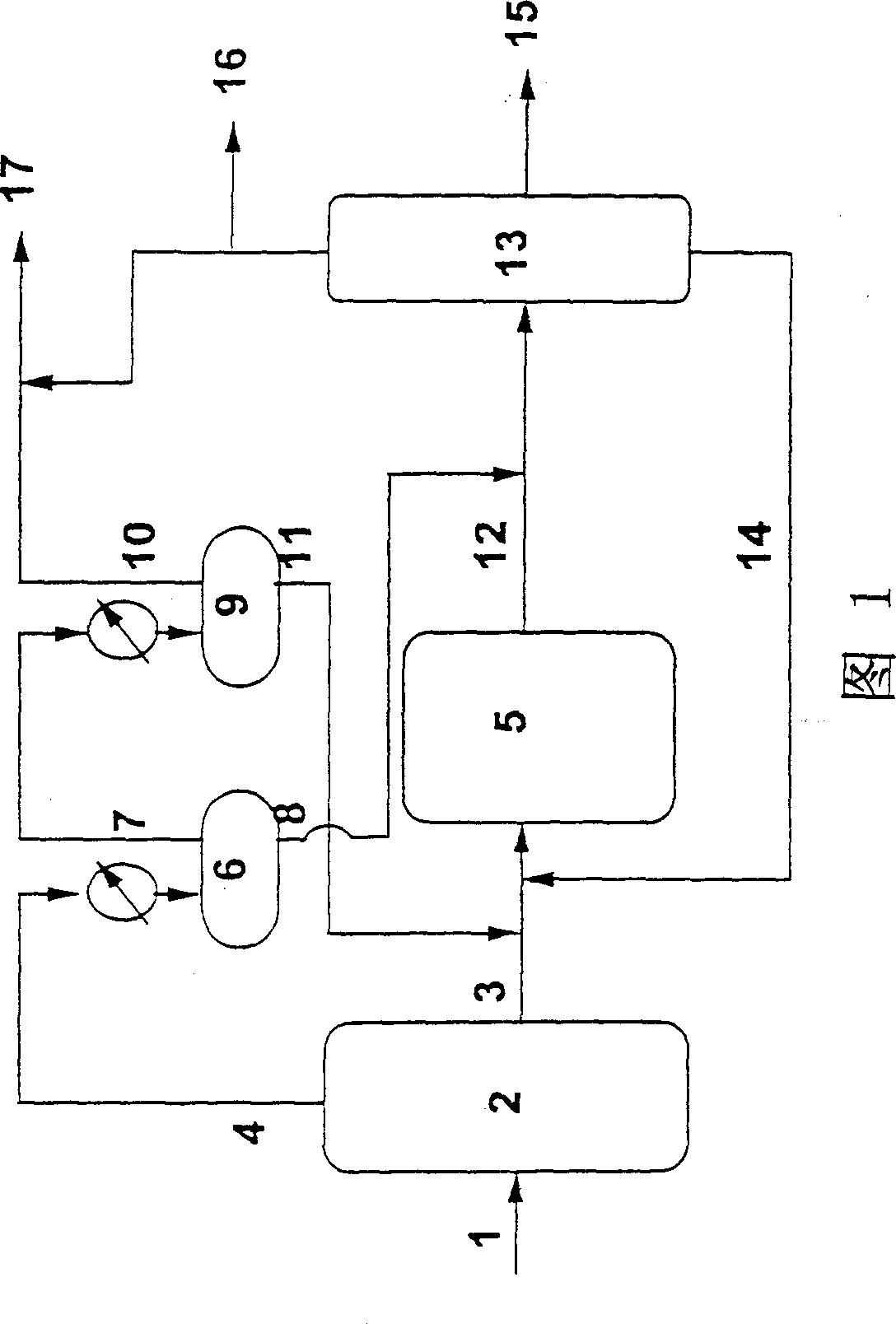
Use of infrared spectroscopy to produce high lubricity, high stability, Fischer-Tropsch diesel fuels and blend fuel
A spectrum and gasoline technology, applied in the distillation control/regulation of hydrocarbon oil, fuel, refined hydrocarbon oil, etc., can solve problems such as fuel corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
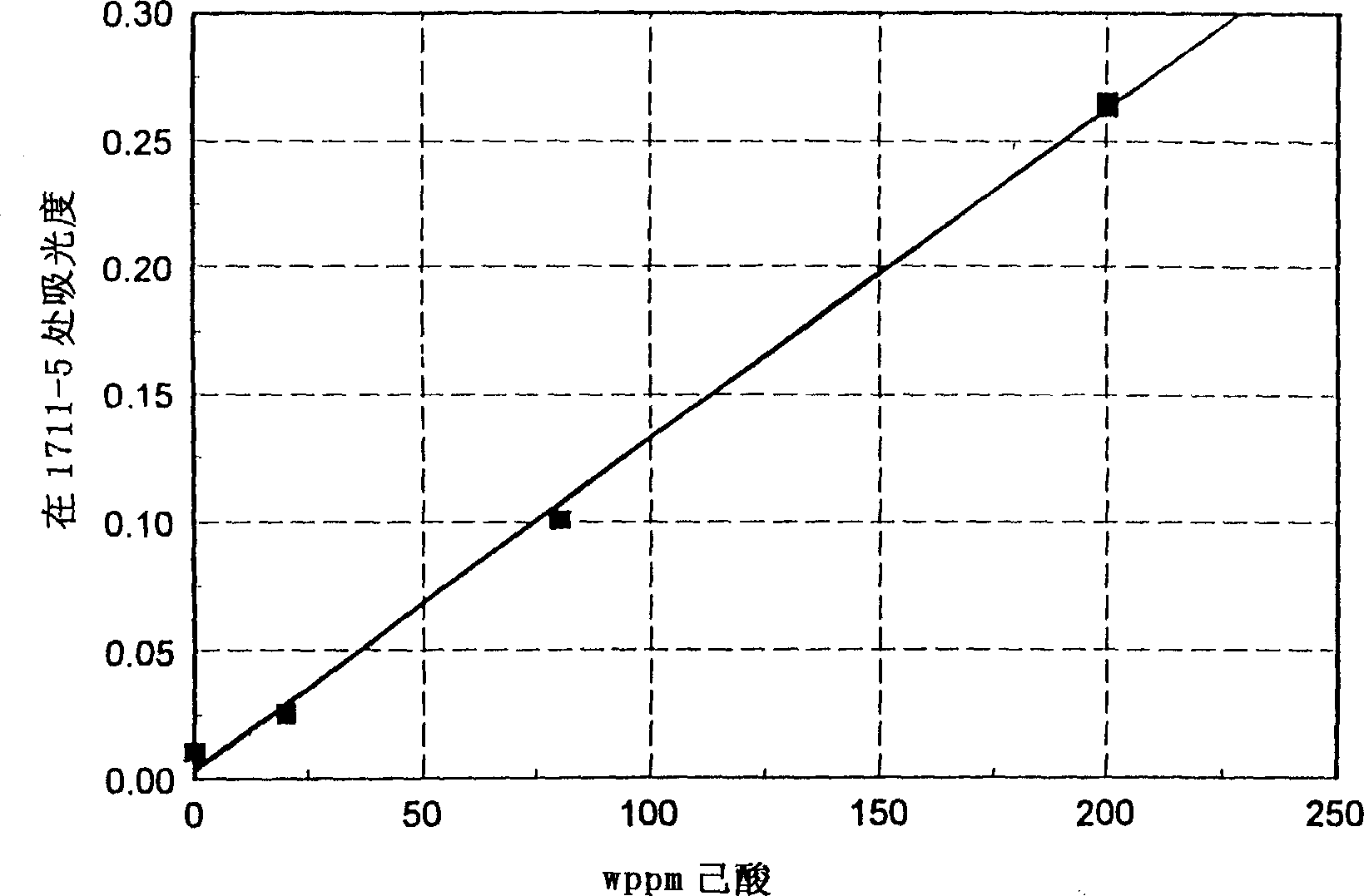
[0019] Hydroisomerized Fischer-Tropsch diesel fuel with a nominal boiling range from 250-700°F was sequentially spiked with 20, 80, and 200 ppm caproic acid. Using a 1mm pathlength sample cell and a 2cm-1 The spectral resolution is measured in the mid-infrared spectrum. Adopt 1755cm -1 and 1685cm -1 The linear corrected baseline drawn between. (Other cell path lengths, spectral resolutions, and baseline correction methods may be used). The peak absorbance is taken at 1711-1715cm -1 The highest absorbance value in the range. The reported absorbance value was determined by measuring the absorbance at the peak maximum relative to the baseline absorbance value at this frequency. figure 2 Show caproic acid concentration and 1713cm -1 The linear relationship of absorbance.
Embodiment 2
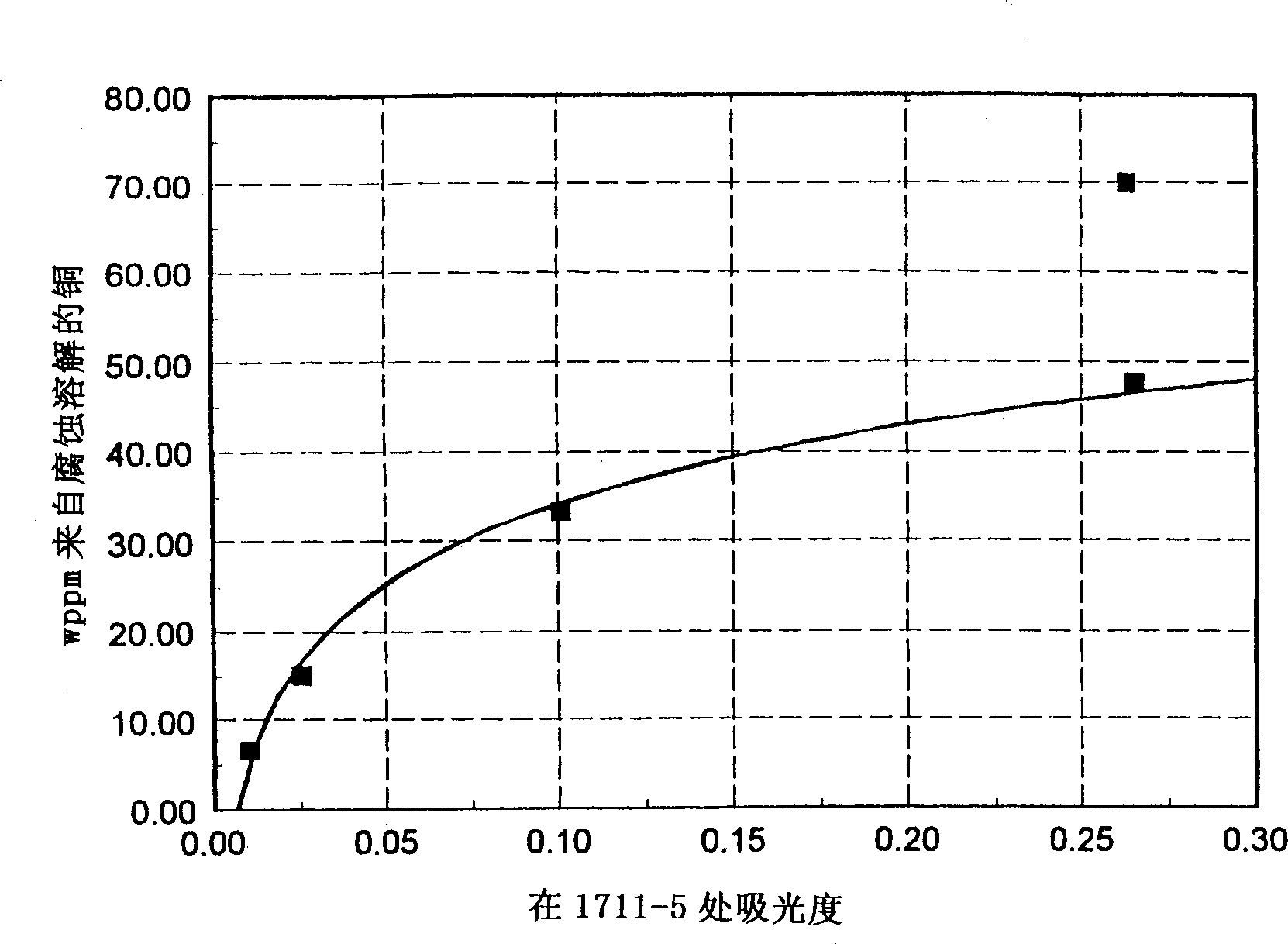
[0021] The following example illustrates a pair of 1713cm -1 Absorbance is monitored to predict fuel corrosivity. Fuel corrosion is measured by the method of ASTM D130 standard copper strip test, with the following improvements: 1) the copper strip must be weighed before and after the experiment to detect the weight loss caused by the corrosion of the sample; 2) after the experiment, the used fuel is tested ICP analysis to detect dissolved (corroded) Cu in solution; 3) The test was performed at 100°C instead of 50°C. The amount of copper corroded into the solution is 1713cm -1 Infrared absorbance plot. It can be clearly seen that 1713cm -1 Absorbance at is very sensitive in predicting the onset of corrosion. If a sample cell with a 1mm optical path length is used, the production process should be adjusted to ensure that the final product is 1713cm -1 The absorbance at is less than 0.05 Å. image 3 shows the solubilized copper pair 1713cm -1 The relationship between IR a...
Embodiment 3
[0023] Hydroisomerized Fischer-Tropsch diesel fuels with nominal boiling ranges from 250-700°F were sequentially spiked with 0.02, 0.1, 0.5 and 1 wt% 1-decene. Using a 1mm pathlength sample cell and a 2cm -1 The spectral resolution is measured in the mid-infrared spectrum. Use 1658cm -1 and 1630cm -1 The linear corrected baseline drawn between. (Other cell path lengths, spectral resolutions, and baseline correction methods may be used). The peak absorbance is taken at 1640-1644cm -1 The highest absorbance value in the range. The reported absorbance value was determined by measuring the absorbance at the peak maximum relative to the baseline absorbance value at this frequency. Figure 4 shows the concentration of 1-decene with 1642cm -1 The linear relationship of absorbance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com