Controlled release peroral compositions of levosimendan
A technology of levosimendan and composition, applied in the field of oral composition, capable of solving problems such as adverse side effects and increased heart rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
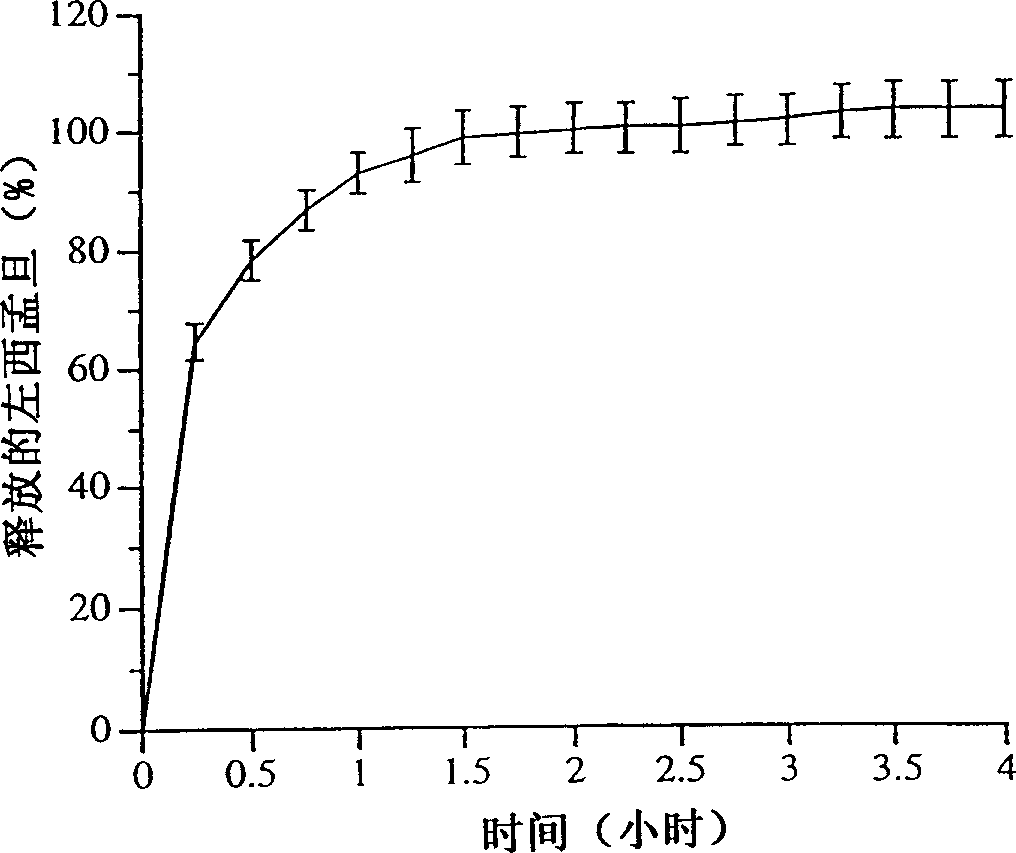
[0029] Example 3 describes a method for determining the in vitro dissolution profile of compositions of the invention.
[0030] Compositions of the invention may be in the form of, for example, tablets, capsules, granules or powders.
[0031] A particularly preferred embodiment of the present invention is obtained by combining an immediate release portion comprising levosimendan and optionally excipients with a controlled release portion comprising levosimendan and a pharmaceutically controlled release component. Drug release control components can be selected as described above, but it is preferred to use hydrophilic gel-forming polymers. A controlled release portion comprising (a) an immediate release portion in powder form comprising levosimendan and at least one excipient, and (b) granular form comprising levosimendan and a controlled release hydrophilic gel-forming polymer Partial compositions are preferred. The immediate release portion in powder form and the controlle...
Embodiment 1
[0042] Example 1. Formulation Examples Formulation 1. Particles: Levosimendan 1.0mg
[0043] Alginic acid 18.0mg
[0044] Methocel K100LV 37.0mg
[0045] Stearic acid 0.6mg Powder part: Levosimendan 1.0mg
[0046] Avicel PH101 84.0mg
[0047] Stearic acid 1.5mg preparation 2. Particles: Levosimendan 1.0mg
[0048] Alginic acid 23.0mg
[0049] Methocel K100LV 46.0mg
[0050] Stearic acid - powder part: levosimendan 1.0mg
[0051] Avicel PH101 69.5mg
[0052] Stearic acid 1.5mg preparation 3. Particles: Levosimendan 1.0mg
[0053] Alginic acid 28.0mg
[0054] Methocel K100LV 56.0mg
[0055] Stearic acid 0.9mg Powder part: Levosimendan 1.0mg
[0056] Avicel PH101 56.0mg
[0057] Stearic acid 1.5mg preparation 4. Particles: Levosimendan 1.0mg
[0058] Alginic acid 33.0mg
[0059] Methocel K100LV 66.0mg
[0060] Stearic acid 0.6mg Powder part: Levosimendan 1.0mg
[0061] Avicel PH101 43.0mg
[0062] Stearic acid 1.5mg
[0063] In the above examples, the granula...
Embodiment 2
[0065] Example 2. Determination of active metabolites (II) in human plasma by liquid chromatography-tandem mass spectrometry
[0066] Preparation of Calibration Samples
[0067]The active metabolite (II) in 20 µl of phosphate buffer pH 7.2 was added to 0.5 ml of analyte-free plasma. Analytes were added in amounts of 0.100, 0.250, 0.500, 1.00, 2.50, 3.75, 5.00, 7.50 and 12.5 ng. After vortexing for 20 seconds, let stand for 10 minutes, add 2500pg internal standard (R)-N-[4-(1,4,5,6-tetrahydro- 4-Ethyl-6-oxo-3-pyridazinyl)phenyl]acetamide. The mixture was vortexed for 1 minute and allowed to stand for 15 minutes. The calibration sample was basified with 50 μl of 0.1 M NaOH and vortexed for 20 seconds. The calibration sample was extracted with 5 ml ethyl acetate:hexane (8:2) by vortexing for 3 minutes. After centrifugation for 7 minutes, the organic layer was separated and concentrated with a Turbo Vap evaporator at 40°C. When the calibration sample was dry, 200 μl of ethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com