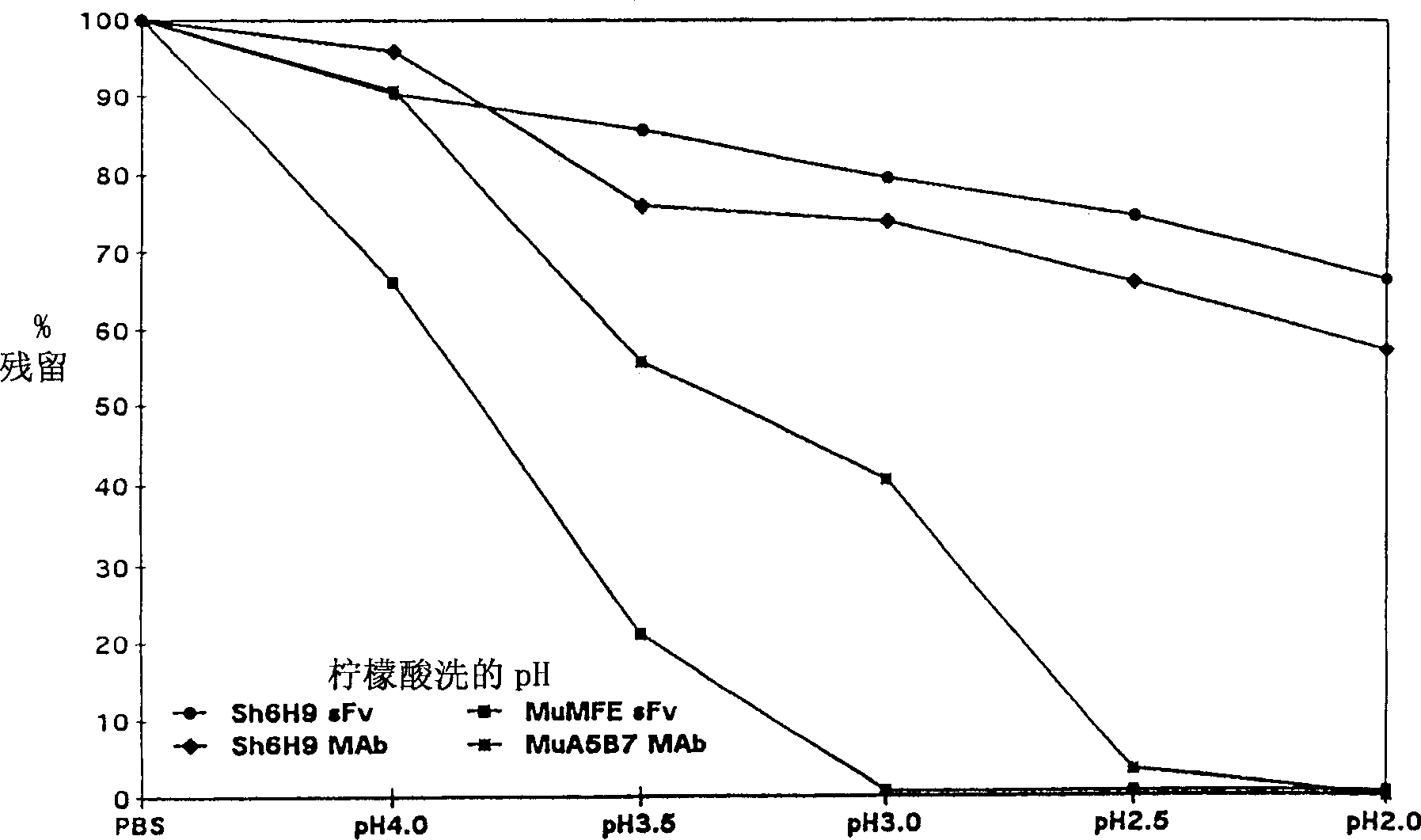
High-affinity antibodies
A kind of affinity and antibody technology, applied in the direction of antibodies, antineoplastic drugs, chemical instruments and methods, etc., can solve the problems of increasing treatment-related costs and unacceptable clinical side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The sheep were immunized with carcinoembryonic antigen (CEA) in complete Freund's adjuvant, and then boosted three times with the antigen in incomplete Freund's adjuvant. Animals were sacrificed and lymph nodes removed after the final booster.
[0029] Lymph node cells were washed and fused with sheep heteromyeloma fusion partner SFP3.2. Fused cells were cultured in medium containing HAT (Life Technologies) at approximately 10 6 The total concentration of cells / m was plated. Then conventional EIA and pickling EIA were used to screen the hybridomas secreting high-affinity antibodies against specific antigens in the samples.
[0030] A standard EIA screening assay is performed as follows:
[0031] The Maxisorb assay plate (NUNC) was coated with CEA (0.4 μg / ml CEA in phosphate buffered saline, pH 7.2), 100 μl per well, overnight at 4°C. Plates were then washed 3 times with phosphate buffered saline pH 7.2 containing 0.01% Tween 20 detergent. Then, 200 μl of pH 7.2 PBS...
Embodiment 2
[0036] Single-chain Fv fragments derived from the hybridoma 6H9 described above were produced as follows:
[0037] mRNA was purified from cultured hybridoma cells using oligo-dT cellulose. Single-stranded DNA (cDNA) complementary to mRNA is synthesized by reverse transcription. Universal primers designed from the heavy and light chain constant regions of sheep antibody genes were used in separate reverse transcription reactions to synthesize antibody variable region cDNA.
[0038] The cDNA is then amplified by polymerase chain reaction and double-stranded DNA is generated using primers designed from the heavy and light chain variable region framework sequences. Separate polymerase chain reactions were used to amplify the heavy and light chain regions. The products were then analyzed by agarose gel electrophoresis, and DNA bands corresponding to the light and heavy chain genes were excised from the gel for purification.
[0039] Equimolar amounts of heavy and light chain var...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com