Use of recombinant human uteroglobin in treatment of inflammatory and fibrotic conditions
A technology of globin and uterus, which is applied in the application field of recombinant human uteroglobin in the treatment of inflammatory and fibrotic diseases, and can solve the problem that uteroglobin cannot inhibit the ECM invasion activity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Recombinant human uteroglobin can be obtained by the method of Mantile et al. (1993). One male and one female P. cynocephalus weighing approximately 400 g were weighed after delivery by hysterotomy (C-section) at 142 days of conception. This is the established model of RDS (Coalson, J.J. et al., The Baboon Model of BPD II: Pathological Features. Exp. Mol. Pathol. 37:355-350 (1982)).
[0163] Following delivery, infants were anesthetized with Ketamine (10 mg / kg) and intubated with a 2.5 mm diameter endotracheal tube. Blood gases and blood pressure were monitored through an arterial line injected percutaneously into the radial artery. A deeper venous line is placed percutaneously into the saphenous vein to infuse fluids, antibodies, and medications. Animals were housed in a servo-controlled infrared incubator, ventilated using a standard time-period, pressure-regulated ventilator with a humidifier, and maintained at 36-37°C. The initial setting is FiO 2 1.0, the rate ...
Embodiment 2
[0167] Embodiment 2. In vitro utilization of soluble PLA 2 Inhibit hydrolysis of artificial surfactants
[0168] In vitro utilization of soluble PLA 2 , rhUG can inhibit the hydrolysis of artificial surfactants. Survanta is an artificial surfactant preparation extracted from the lungs of cattle and is indicated for the treatment of preterm neonates with respiratory distress syndrome (ARDS) and adults with respiratory distress syndrome (ARDS). Survanta is the first group of soluble PLA 2 Hydrolyzed, such as porcine, pancreatic PLA 2 , characterized as a substrate with the ability to compete with the fluorescent lecithin substrate and produce arachidonic acid product.
[0169] Survanta is an in vitro substrate that can be treated by the first group of soluble PLA 2 degradation. In vitro, Survanta can be rapidly absorbed by PLA in the extracellular fluid of human lungs 2 degradation. RhUG can inhibit the degradation of Survanta in vitro. Embodiment 3. Construction of u...
Embodiment 3
[0169] Survanta is an in vitro substrate that can be treated by the first group of soluble PLA 2 degradation. In vitro, Survanta can be rapidly absorbed by PLA in the extracellular fluid of human lungs 2 degradation. RhUG can inhibit the degradation of Survanta in vitro. Embodiment 3. Construction of uteroglobin knockout mice
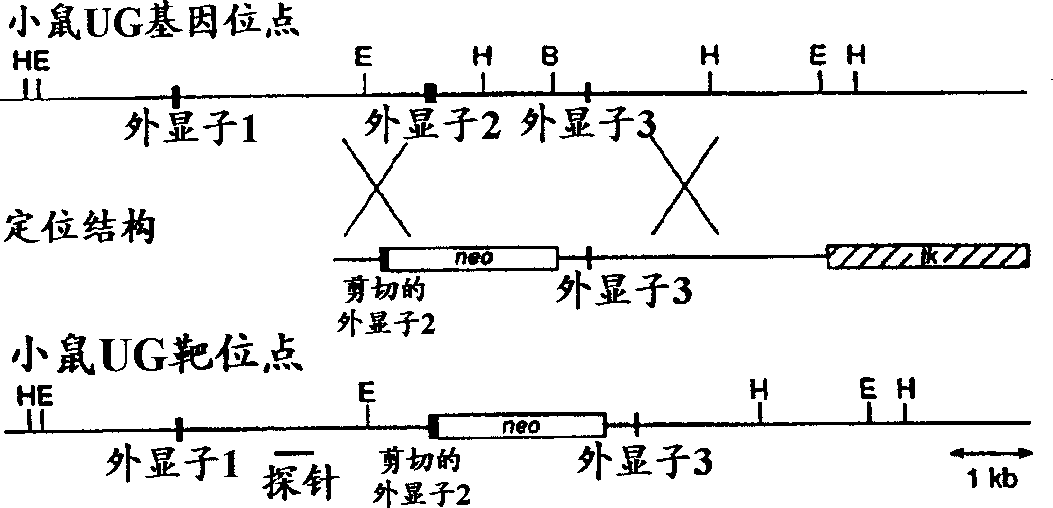
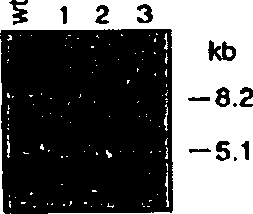
[0170] The purpose of establishing a transgenic uteroglobin knockout mouse was to determine the role of uteroglobin in mammalian physiology and to generate a model of uteroglobin therapy in several inflammatory clinical diseases. The first step was to construct an appropriate DNA vector with which the endogenous uteroglobin gene in mice could be targeted and blocked. A 3.2 kb BamHI-EcoRI DNA fragment derived from the uteroglobin agent gene of the 129 / SVI mouse strain (RAY, 1993) containing exon 3 and flanking sequences was subcloned into p PNW The corresponding site of the vector, as described by Lei et al. (1996). Transform the NotI and XhoI res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com