Methyl or ethyl protodioscin chemical synthesis method
A technology of diosgenin and ethyl yam, applied in organic chemistry, steroids, etc., can solve the problems of limiting structure-activity relationship research, difficulty in separation and purification, and low content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The following examples will help to understand the present invention, but do not limit the content of the present invention.
[0037] Synthesis Example 1
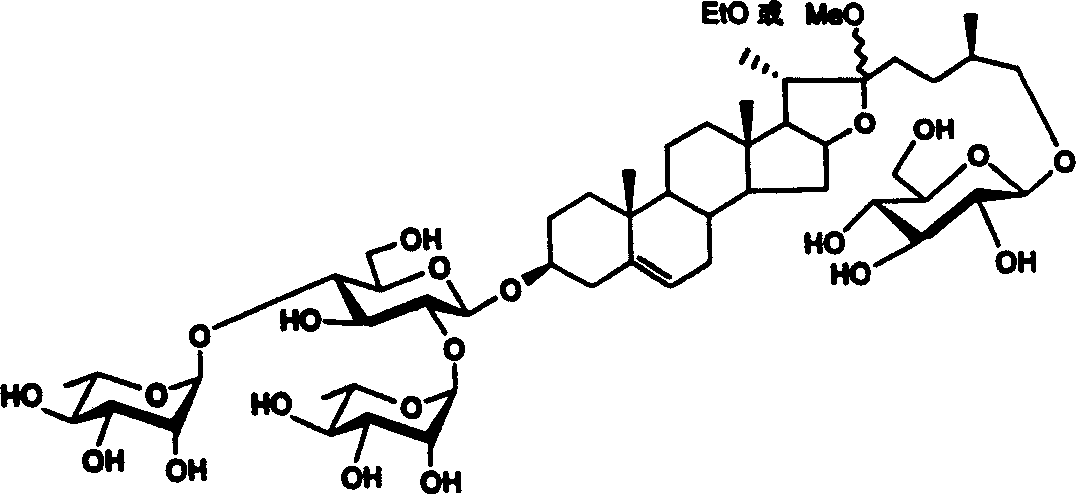
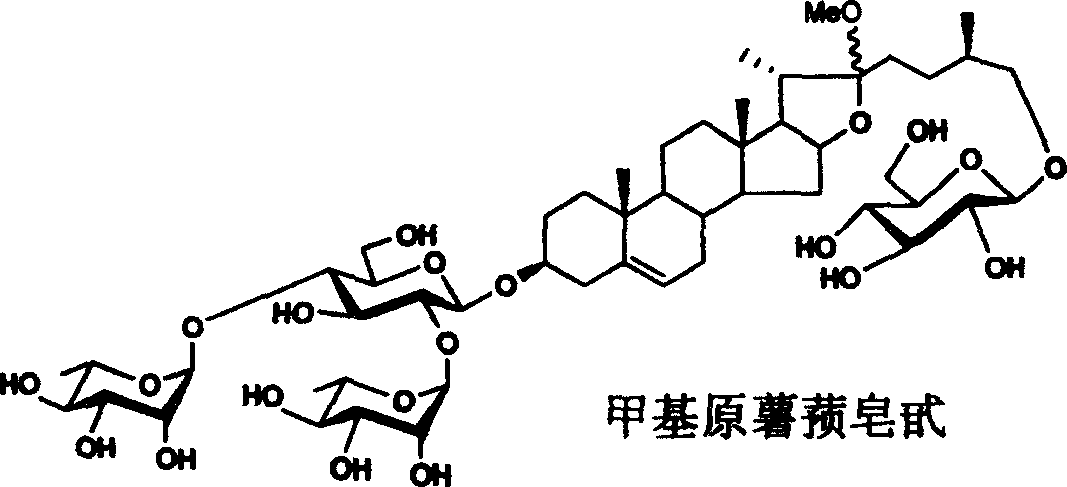
[0038] Synthesis of methyl protodioscin:
[0039]
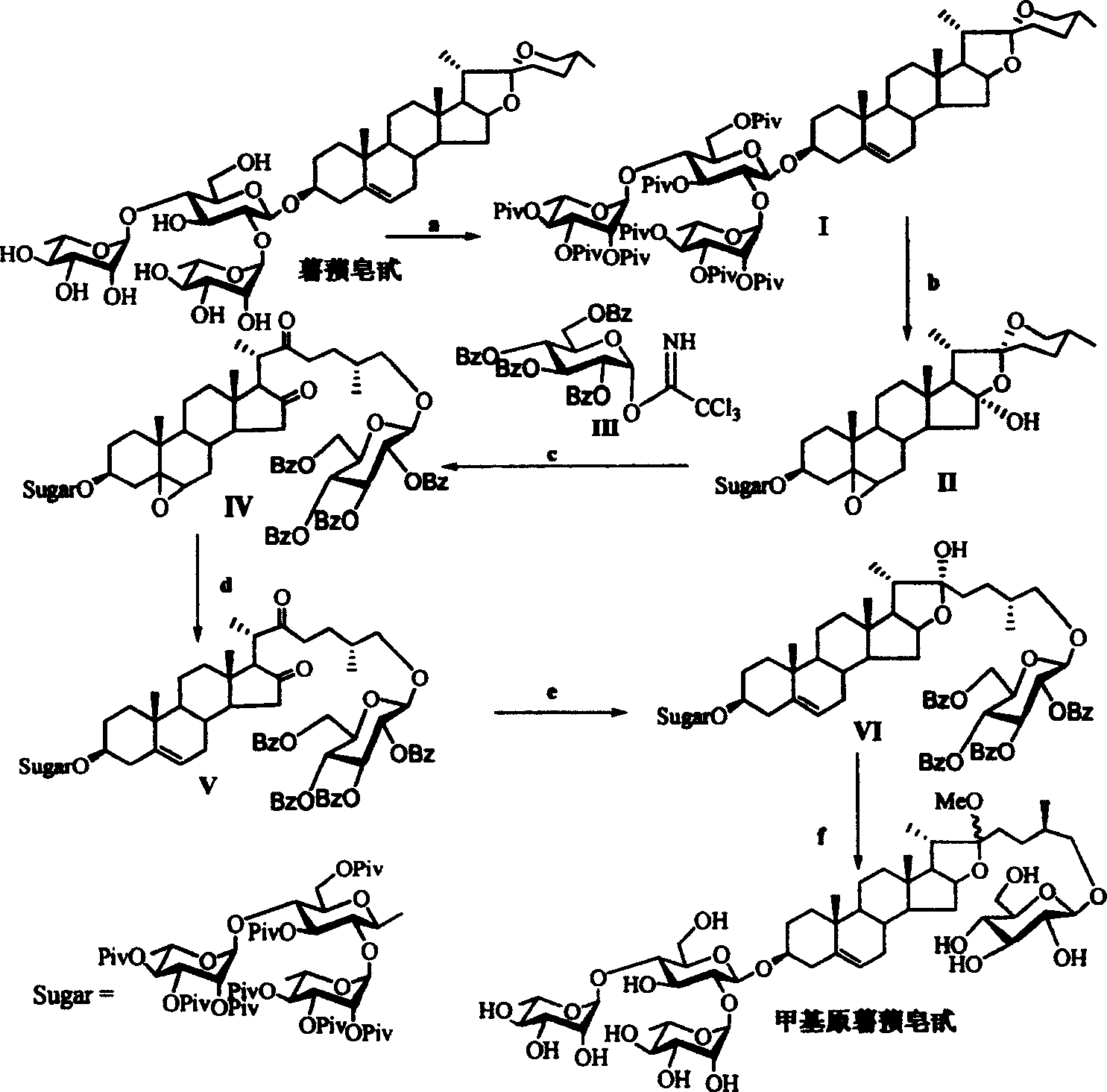
[0040] Reaction formula 1: specific synthetic route and embodiment of methyl protodioscin
[0041] Reagents and conditions: a) 2,2,2-tri-methylacetyl chloride (PivCl), pyridine, room temperature; b) potassium persulfate preparation, acetone / H 2 O / C1-C6 halogenated hydrocarbon; c)III, dehydrating agent, accelerator, CH 2 Cl 2 ; d) (C 1~6 h 3~13 ) 3 SiI, NaI, CH 3 CN; e) NaBH 4 , i-PrOH, f) NaOH, CH 3 OH, H 2 o
[0042] (1) Reaction of dioscin with 2,2,2-tri-methylacetyl chloride (PivCl) in pyridine to obtain fully protected dioscin I; (2) oxidation of dioscin I with oxone to obtain double bond and 16-position oxidized Product II; (3) under glycosylation conditions, oxidation product II reacts with benzoyl (Bz)-protected glucose trisimide ester donor II...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com