Process for nitrous oxide purification
A nitrous oxide, carbon monoxide technology, applied in the directions of nitrous oxide, nitrous oxide capture, chemical instruments and methods, etc., can solve the problems of pollution of nitrous oxide, waste, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0027] A kind of method of purifying nitrous oxide gas of the present invention is carried out as follows:
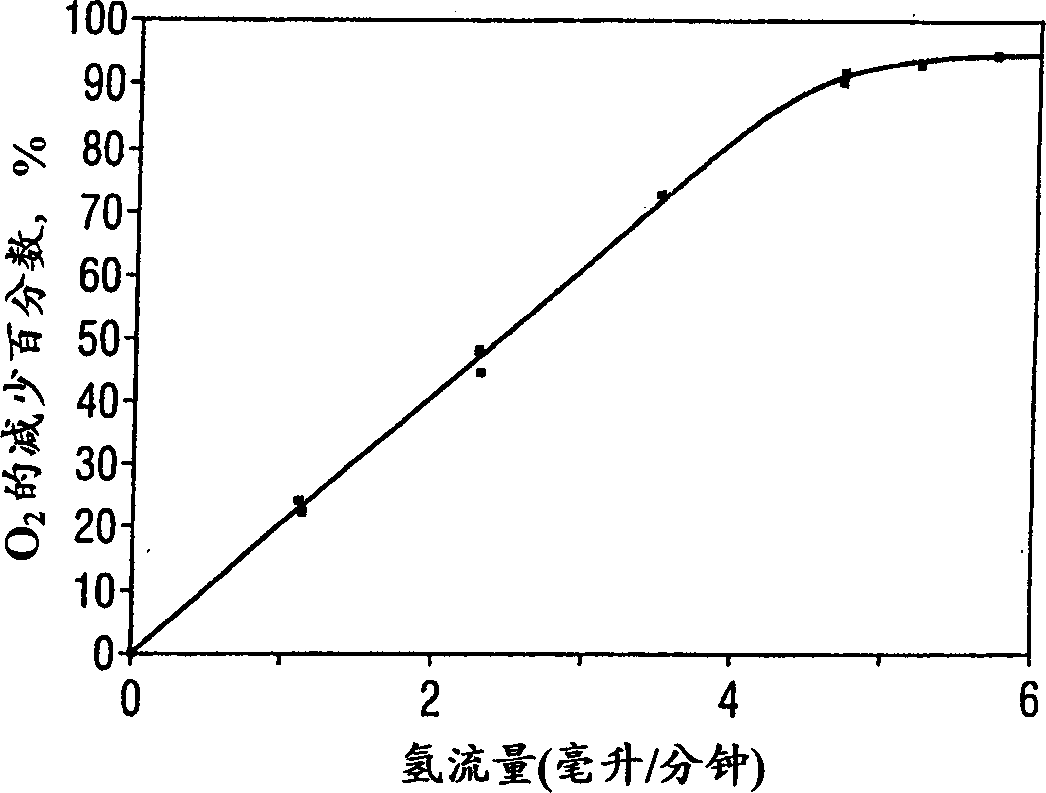
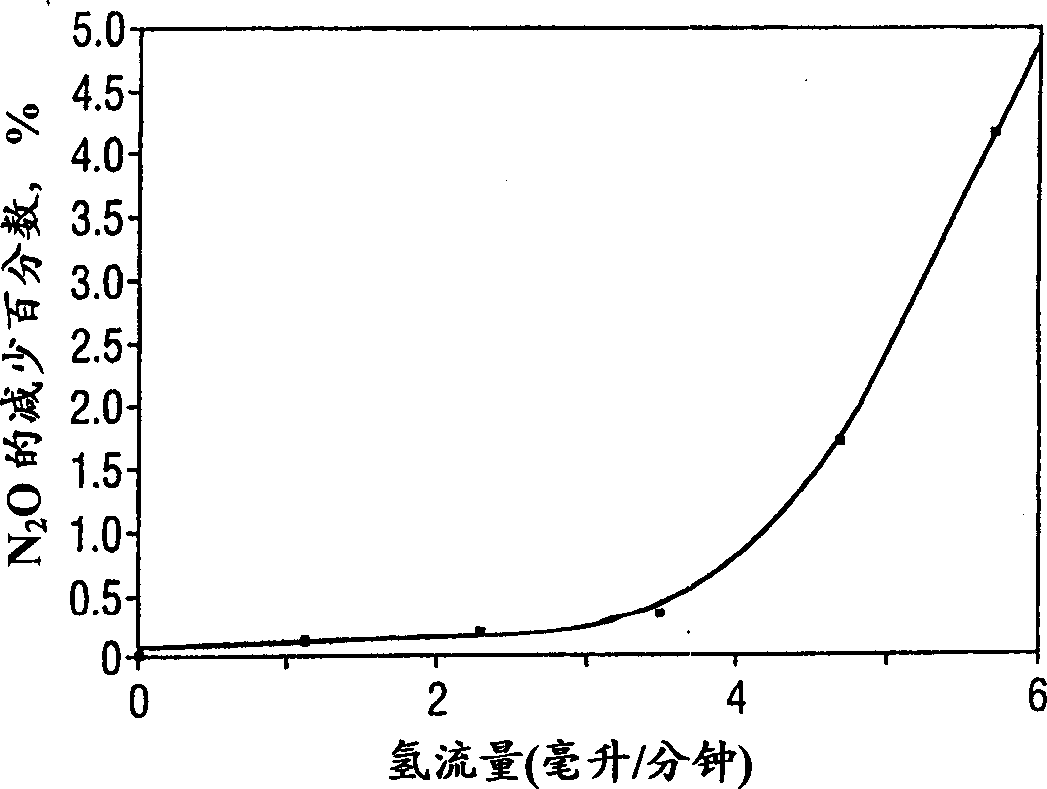
[0028] 0.2% (w / w) palladium on a beaded alumina catalyst from Johnson-Matthey was exposed to 60 cubic centimeters per minute (containing 2.4 cubic centimeters of oxygen at 36 cubic centimeters of nitrous oxide and 6 cubic centimeters of helium). The contact time was 4.6 seconds. Part of the helium is gradually replaced by pure hydrogen. Reaction Tracking Reactor effluents were analyzed by mass spectrometry (oxygen atomic mass units 32, 28, 30 and nitrous oxide atomic mass units 44, hydrogen atomic mass units 2, water atomic mass units 18). Complete hydrogen reaction was observed in all test examples. figure 1 and figure 2 The selective reaction of oxygen with hydrogen but not with nitrous oxide, respectively, is shown. figure 1 showing the destruction of oxygen as a function of hydrogen flow, figure 2 Shows the percent reduction in nitrous oxide as a function of...
Embodiment II
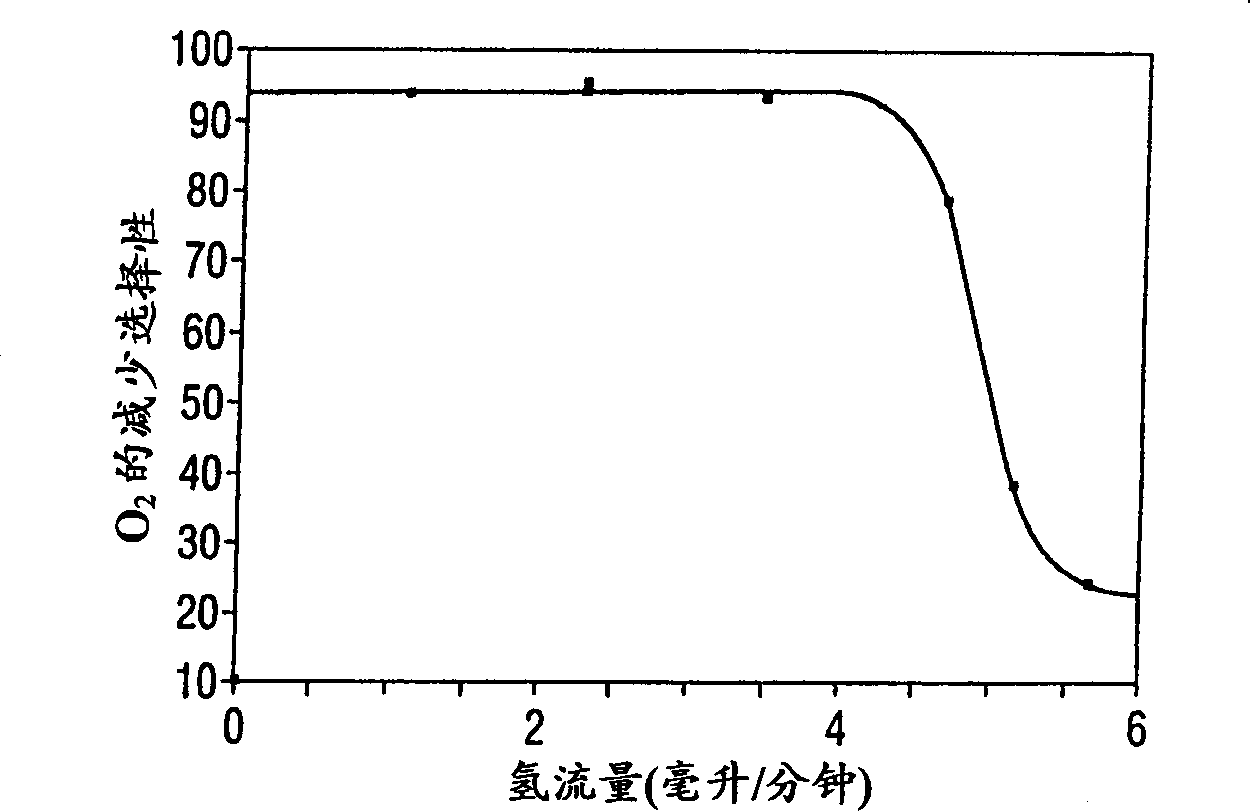
[0030] The same experiment was carried out at 25°C with a contact time of 0.7 seconds and the gas flow composition and rate as before. Figure 4 and Figure 5 shows the performance obtained under this condition. Figure 4 shows the reduction of oxygen as a function of hydrogen flow, Figure 5 The percent reduction in nitrous oxide as a function of hydrogen flow is shown. Image 6 The selectivity of oxygen reduction as a function of hydrogen flow is demonstrated.
Embodiment III
[0032] The same experiment was carried out at 25°C with a contact time of 0.7 seconds and the gas flow composition and rate as before. But part of the hydrogen is replaced by CO (ie nearly 50% by volume of CO). The results in terms of oxygen reduction and selectivity are the same as those obtained with pure hydrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com