New pyrimidine-2,4,6-trione derivatives, processes for their production and pharmaceutical agents cnotaining these compounds
A compound, pyrimidine technology, applied in the field of pyrimidine-2, can solve the problem of low oral effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
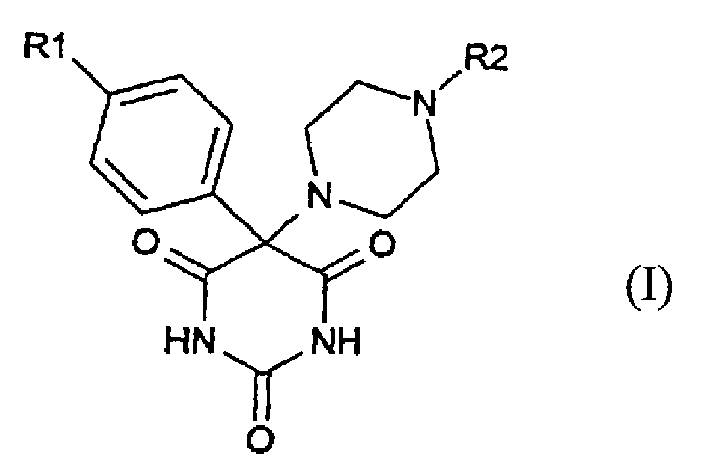
[0039] 5-(4-(4-Chloro-phenoxy)-phenyl)-5-(4-pyrimidin-2-yl-piperazine)-pyrimidine-2,4,6-trione
[0040] A) 1-(4-(4-chloro-phenoxy)-phenyl-ethanone
[0041] 4-Fluoro-acetophenone (24.4 g) was dissolved in dimethylformamide (180 ml), and 4-chlorophenol (22.8 g) and potassium carbonate (29.5 g) were added. The mixture was heated at reflux for 7 hours with stirring. After cooling, the mixture was diluted with water and extracted with dichloromethane. The organic phase was washed with water, dried and evaporated to give 38 g of a crystalline solid. M.p.66-68°C.
[0042] B) 2-(4-(4-chloro-phenoxy)-phenyl)-morpholin-4-yl-ethanthione
[0043] 12.4 g of the product obtained by the above procedure were mixed with sulfur (4 g) and morpholine (8.8 ml). The mixture was heated to 150°C for 2 hours, cooled in an ice bath and treated with ethanol (20ml) for 30 minutes. The precipitated crystals were collected and recrystallized from ethanol to obtain 13 g of the title compound. m.p.104...
Embodiment 2
[0057] 5-[4-(4-Chloro-phenoxy)-phenyl]-5-(2,3,5,6-tetrahydro-[1,2']bispyrazinyl-4-yl)-pyrimidine -2,4,6-trione
[0058] Similar to Example 1, step H, the title compound was prepared using 330 mg of 1-(pyrazin-2-yl)-piperazine instead of N-(pyrimidin-2-yl)-piperazine to obtain 460 mg of title compound. Determined by mass spectrometry: m / e: 492.
Embodiment 3
[0060] No.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com