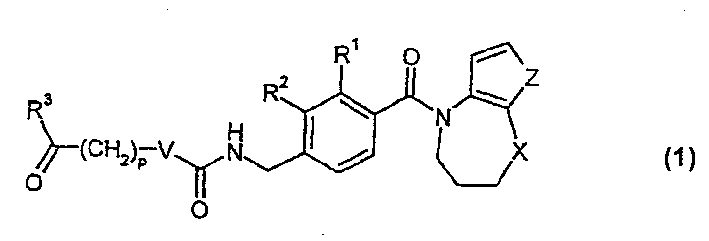
Bicycle vasporession agonists
A compound and selected technology, applied in the direction of blood diseases, active ingredients of heterocyclic compounds, extracellular fluid diseases, etc., can solve the problems of low oral bioavailability, poor water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A4
[0061] Example A 4-(tert-Butoxycarbonylaminomethyl)-3-chlorobenzoic acid A1. Methyl 4-bromomethyl-3-chlorobenzoate
[0062] To the solution of methyl 3-chloro-4-methylbenzoate (5.0g, 27.1mmol) in carbon tetrachloride (50ml) was added NBS (5.8g, 32.0mmol) and AIBN (0.442g, 2.70mmol) and stirred The mixture was refluxed for 18 hours. The mixture was allowed to cool to room temperature and then concentrated under vacuum. The residue was purified by flash silica gel chromatography (eluent: ethyl acetate: petroleum ether=0:100-5:95), yield = 5.96 g (84%). A2. 4-(tert-Butoxycarbonylaminomethyl)-3-chlorobenzoic acid
[0063] To a saturated ethanol solution (170 ml) of ammonia was added methyl 4-bromomethyl-3-chlorobenzoate (5.5 g, 20.9 mmol) obtained in Example A1, and the mixture was stirred at room temperature for 1 hour, and then Concentrate under vacuum. Triturate the residue with ether, filter the white crystals obtained, and wash with more ether. Add (BOC) to the solid water (100m...
Embodiment B4
[0064] Example B 4-cyano-3-methylbenzoic acid
[0065] Under a nitrogen atmosphere at -78°C, to a solution of 4-bromo-2-methylbenzonitrile (2.0g, 10.2mmol) in THF (100ml) was added dropwise a 2.5M solution of n-butyllithium (4.48ml, 11.2mmol) mmol). The mixture was stirred at -78°C for 1 hour, then poured onto solid carbon dioxide (5 g) in THF (50 ml), and the mixture was warmed to room temperature. Water (200 ml) was added, and the mixture was extracted three times with ether. Concentrated hydrochloric acid was added to acidify the aqueous layer and extracted three times with chloroform. The combined chloroform extracts were washed with water, dried over magnesium sulfate, and concentrated under vacuum to obtain a white solid, yield = 1.2 g (73%).
Embodiment C
[0066] Example C4-cyano-2-methylbenzoic acid
[0067] The 4-bromo-3-methylbenzonitrile (2.0g, 10.2mmol) was reacted according to the method of Example B to obtain a yellow solid, which was triturated with hexane and filtered. Yield=0.96g (59%) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com