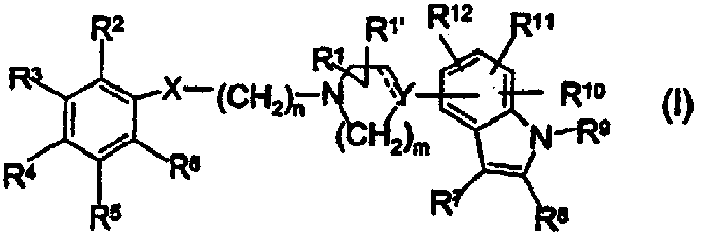
Novel indole derivatives
A technology of indole and compound, applied in the field of new indole derivatives, can solve the problem of lack of extrapyramidal activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] 1a. 4-{4-[3-(2-Chloro-phenoxy)-propyl]-piperazin-1-yl}-1H-indole
[0175] To a slurry of sodium hydride (47 mmol) in tetrahydrofuran (50 mL) was added dropwise a solution of 2-chlorophenol (5 g) in tetrahydrofuran (25 mL) at room temperature. The mixture was stirred for 30 minutes. The reaction mixture was warmed to reflux, after which 2-bromo-1-propanol (3.5 mL) in tetrahydrofuran (25 mL) was added over 5 minutes. The mixture was refluxed overnight, another equivalent of 3-bromo-1-propanol was added, and the mixture was refluxed for an additional 12 hours. The mixture was cooled, brine and ethyl acetate were added and washed using standard procedures. The combined organic phases were dried and evaporated. The crude product 3-(2-chlorophenoxy)-1-propanol was dissolved in acetonitrile (500 mL), and carbon tetrabromide (38.7 g) was added. To the cooled (0° C.) mixture was added triphenylphosphine (25.5 g) portionwise over 30 minutes. The reaction was allowed to proce...
Embodiment 2
[0177] 2a. 4-{4-[3-(2-Chloro-phenylthio)-propyl]-piperazin-1-yl}-1H-indole, 0.75 oxalate
[0178]To a slurry of sodium hydride (38 mmol) in dimethylformamide was added a solution of 2-chlorothiophenol (5 g) in dimethylformamide (50 mL) dropwise over 15 minutes at room temperature. The mixture was stirred for 30 minutes. The reaction mixture was added slowly (10 minutes) to a solution of 1,3-dibromopropane in dimethylformamide (25 mL) at room temperature. The final mixture was stirred for an additional 60 minutes. The reaction was quenched by adding sufficient water to consume excess sodium hydride, acidified with etherified hydrochloric acid, and evaporated. The crude product was purified by flash chromatography on silica gel (heptane:ethyl acetate:triethylamine / 95:2.5:2.5) to give 3-(2-chlorophenylthio)-1-propyl bromide (5.7 g). (1H-indol-4-yl)piperazine (1.1 g), potassium carbonate (2.3 g), potassium iodide (catalyst ) and 3-(2-chlorophenylthio)-1-propyl bromide (1.5 g) ...
Embodiment 3
[0190] 3a. 4-{4-[2-(2-Chloro-4-fluoro-phenylthio)-ethyl]-piperazin-1-yl}-1H-indole, 1.25 oxalate
[0191] To a mixture of (1H-indol-4-yl)piperazine (2.50 g) and triethylamine (3.8 g) in dry tetrahydrofuran was added dropwise a solution of chloroacetyl chloride (1.86 g) over 10 minutes at room temperature. Solution in water tetrahydrofuran (5 mL). After 40 minutes the reaction was quenched with water and washed (ethyl acetate) using standard procedures. Drying and evaporation gave 3.5 g of the chloroacetylated derivative. This crude product was used directly in the next step. 2-Chloro-4-fluorothiophenol (1.1 g) was dissolved in tetrahydrofuran (40 mL), and potassium tert-butoxide (0.84 g) was added, followed by stirring for 10 minutes. The above mixture was treated dropwise with a solution of the chloroacetylated derivative prepared above (1.70 g) in tetrahydrofuran (20 mL). The reaction was allowed to proceed at room temperature for 1 hour, then at reflux for 20 minutes be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com