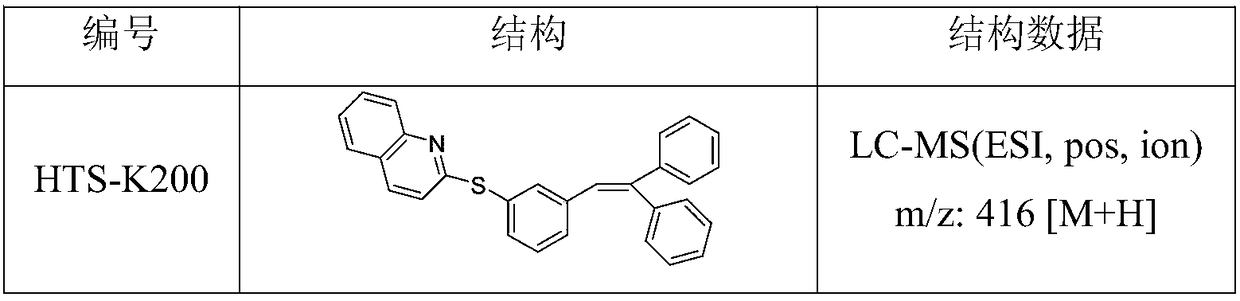
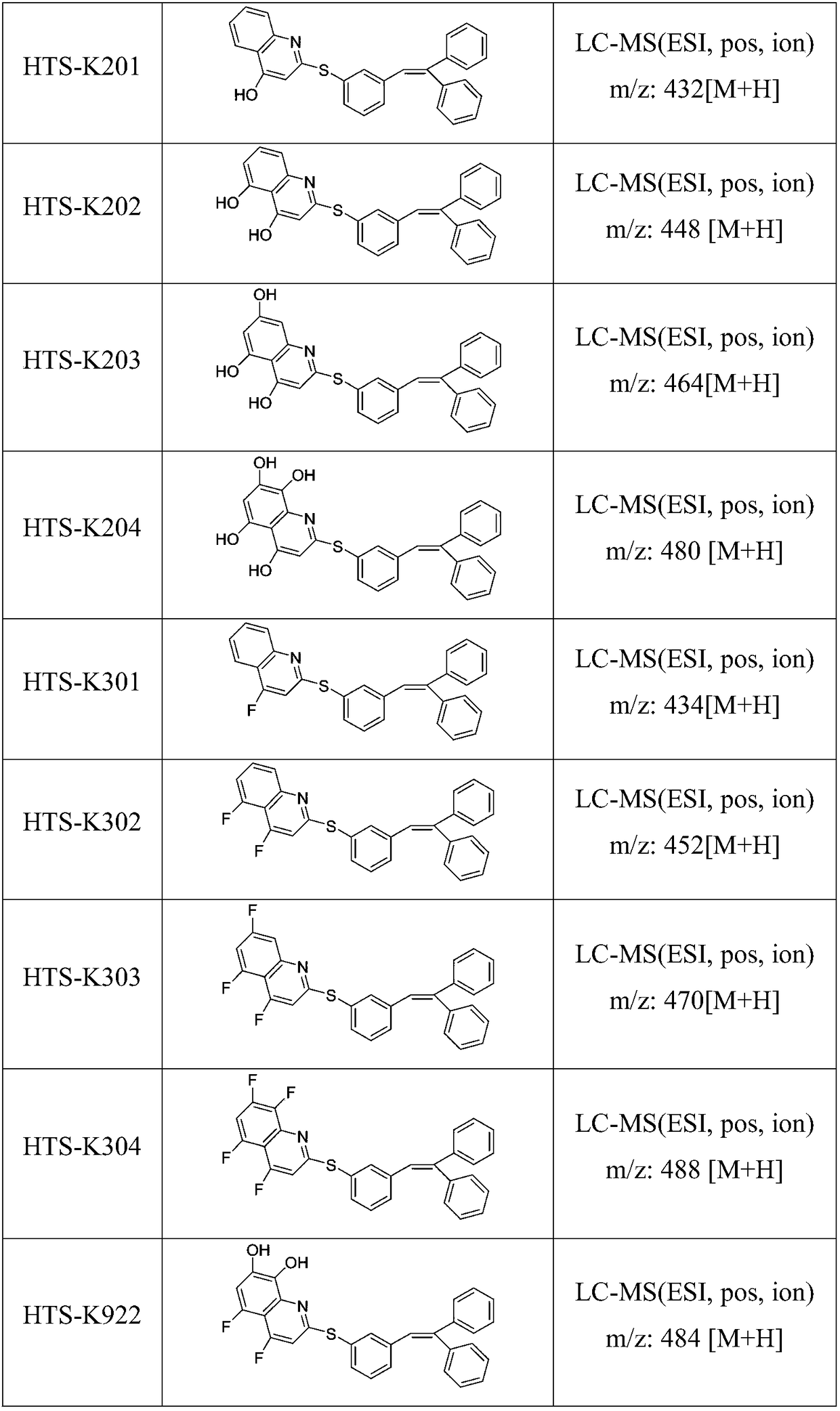
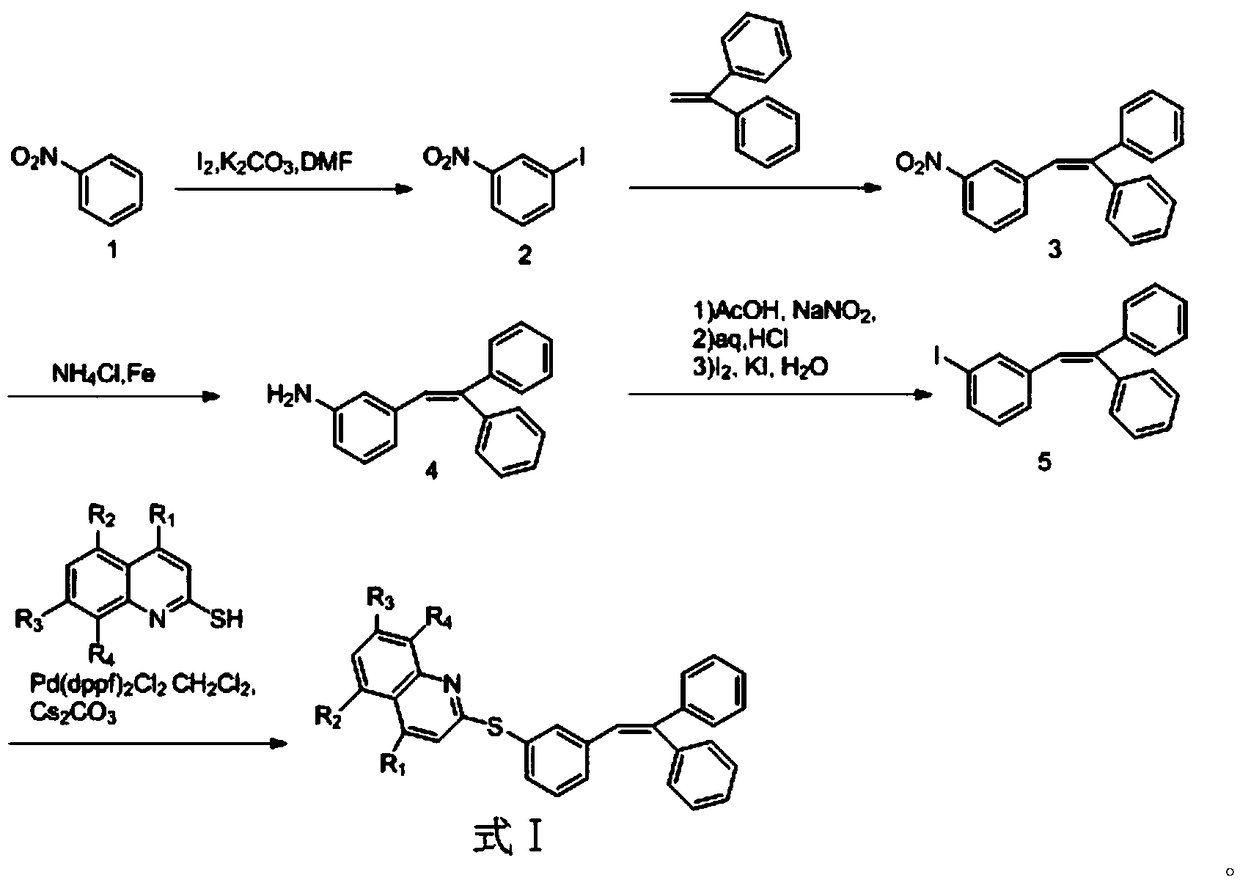
Compound having dual functions to 5-HT and application thereof in depressive disorder treatment
A compound and solvate technology, applied in the field of application in the treatment of depression, can solve problems such as sexual dysfunction, low efficacy, suicidal tendencies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of (E)-2-((3-(2,2-diphenyl-vinyl)phenyl)thio)quinoline
[0029] Synthesis of 1-1, 1-iodo-3-nitrobenzene:
[0030]
[0031] Add 70mL of DMF in a 500mL reaction flask, then add nitrobenzene (22.5g, 0.183mol) and potassium carbonate (25.2g, 0.182mol), and cool in an ice bath below 10°C. After stirring, iodine (41.6g, 0.164mol) was dissolved in 30mL DMF and added dropwise to the reaction system for 1 hour. The reaction mixture was stirred at 25°C for 16 hours until the reaction was complete, and sodium thiosulfate (22.3g, 0.141mol) and potassium carbonate were added (0.150g) in aqueous solution (150mL), while maintaining the internal temperature below 30°C, stirred for 30 minutes, added 200mL of water while stirring, solid precipitated, filtered with suction, washed the filter cake with water, and vacuum dried at 60°C for 12 hours to obtain a light yellow solid 1-iodo-3-nitrobenzene, 43.84g, yield 96.2%. 1 H-NMR (400MHz, CDCl3) δ: 7.35(t, 1H), 8.15...
Embodiment 2
[0044] Example 2: Synthesis of (E)-2-((3-(2,2-diphenyl-vinyl)phenyl)thio)-4-hydroxyl-quinoline:
[0045]
[0046] Add 175mL DMF and (E)-1-iodo-3-(2,2-diphenyl-vinyl)benzene (38.78g, 0.102mol) into a 500mL reaction flask, nitrogen protection, add [1,1'-bis (Diphenylphosphine)ferrocene]dichloropalladium dichloromethane complex (1.49g, 0.0018mol), cesium carbonate (19.8g, 0.061mol) and dichloromethane 2mL, add 4-hydroxy-quinoline -2-thiol (0.105mol), heated to 80°C, reacted for 16 hours until the reaction was complete, removed DMF by rotary evaporation, cooled to room temperature, added 100mL of ethyl acetate, 150mL of water, stirred for 40 minutes, separated the organic phase, brine Wash, separate layers, dry the organic phase with sodium sulfate, filter, rotary evaporate to dryness, and flash column chromatography to obtain light yellow (E)-2-((3-(2,2-diphenyl-vinyl)phenyl )thio)-4-hydroxy-quinoline, 34.29g, yield 78%. LC-MS (ESI, pos, ion) m / z: 432 [M+H].
Embodiment 3
[0047] Example 3: Synthesis of (E)-2-((3-(2,2-diphenyl-vinyl)phenyl)thio)-4-fluoro-quinoline:
[0048]
[0049] Add 175mL DMF and (E)-1-iodo-3-(2,2-diphenyl-vinyl)benzene (38.78g, 0.102mol) into a 500mL reaction flask, nitrogen protection, add [1,1'-bis (Diphenylphosphine)ferrocene]dichloropalladium dichloromethane complex (1.49g, 0.0018mol), cesium carbonate (19.8g, 0.061mol) and dichloromethane 2mL, add 4-fluoro-quinoline -2-thiol (0.105mol), heated to 80°C, reacted for 16 hours until the reaction was complete, removed DMF by rotary evaporation, cooled to room temperature, added 100mL of ethyl acetate, 150mL of water, stirred for 40 minutes, separated the organic phase, brine Wash, separate layers, dry the organic phase with sodium sulfate, filter, rotary evaporate to dryness, and flash column chromatography to obtain light yellow (E)-2-((3-(2,2-diphenyl-vinyl)phenyl )thio)-4-fluoro-quinoline, 34.89g, yield 79%. LC-MS (ESI, pos, ion) m / z: 434 [M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com