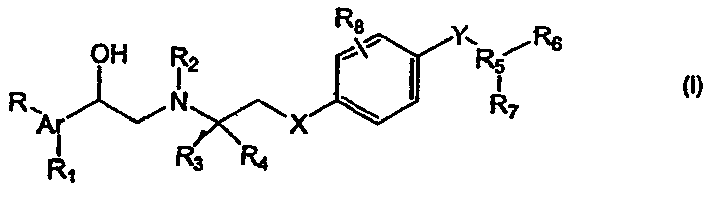
Alpha-aryl ethanolamines and their use as beta-3 adrenergic receptor agonists
A technology of ethanol and alkyl, applied in anti-inflammatory agents, antiviral agents, non-central analgesics, etc., can solve problems such as unclear mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] {2-[-4-(4-Methyl-oxazol-2-yl)-phenoxy]-ethyl}-carbamic acid benzyl ester
[0144] [2-(4-Carbamoyl-phenoxy)-ethyl]-carbamate benzyl ester (322mg, 1.02mmol) and 1-bromo-2,2-dimethoxypropane (3.8g, 20.4mmol ) were combined in a round bottom flask and heated at about 130°C for about 30 minutes. The reaction mixture was then cooled to room temperature and poured into water. The mixture was extracted with ethyl acetate, and the combined extracts were dried over magnesium sulfate, filtered and concentrated in vacuo. The resulting crude product was purified by column chromatography (1:1 hexane / ethyl acetate) to obtain the desired oxazole product (167 mg, 47% yield). LRMS([M+H] + ) = 353.1.
Embodiment 2
[0146] 2-[4-(4-Methyl-oxazol-2-yl)-phenoxy]-ethylamine
[0147] The title compound {2-[4-(4-methyl-oxazol-2-yl)-phenoxy]-ethyl}-carbamic acid benzyl ester (166 mg, 0.47 mmol) of Example 1 was dissolved in methanol ( 5 ml), 10% Pd / C (50 mg) and 1,4-cyclohexadiene (192 mg, 2.4 mmol) were added to the resulting solution. The mixture was stirred for about 16 hours, then filtered through celite, washing the filter pad with methanol. Concentrate the filtrate to dryness in vacuo, 1 The resulting product (92 mg, 90% yield) as determined to be pure compound by H NMR was used without further purification. LRMS([M+H] + ) = 219.2.
Embodiment 3
[0149] 4-Hydroxythiobenzamide
[0150] In a round bottom flask, 4-hydroxybenzonitrile (5.00 g, 41.9 mmol), diethylthiophosphoric acid (7.02 g, 41.9 mmol) and water (8 ml) were heated at about 80° C. for about 30 minutes with stirring. A further 10 ml of water were then added to the suspension, and the solution was heated for a further approximately 1 hour. The mixture was stirred at room temperature for about 16 hours and extracted with water and 1:1 ether / ethyl acetate. The combined organic extracts were dried over magnesium sulfate, filtered and concentrated in vacuo. The resulting solid was purified by column chromatography (silica gel; hexane to ethyl acetate). The product was isolated as a yellow solid (5.54 g, 87% yield). 1 H NMR (CD 3 OD): δ 6.74 (d, 2H, J = 9.1 Hz), 7.83 (d, 2H, J = 8.7 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com