Patents
Literature
46 results about "Beta-3 adrenergic receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The beta-3 adrenergic receptor (β₃ adrenoreceptor), also known as ADRB3, is a beta-adrenergic receptor, and also denotes the human gene encoding it.
Phenylethanolamine compounds useful as beta 3 agonists, process for producing the same, and intermediates in the production of the same
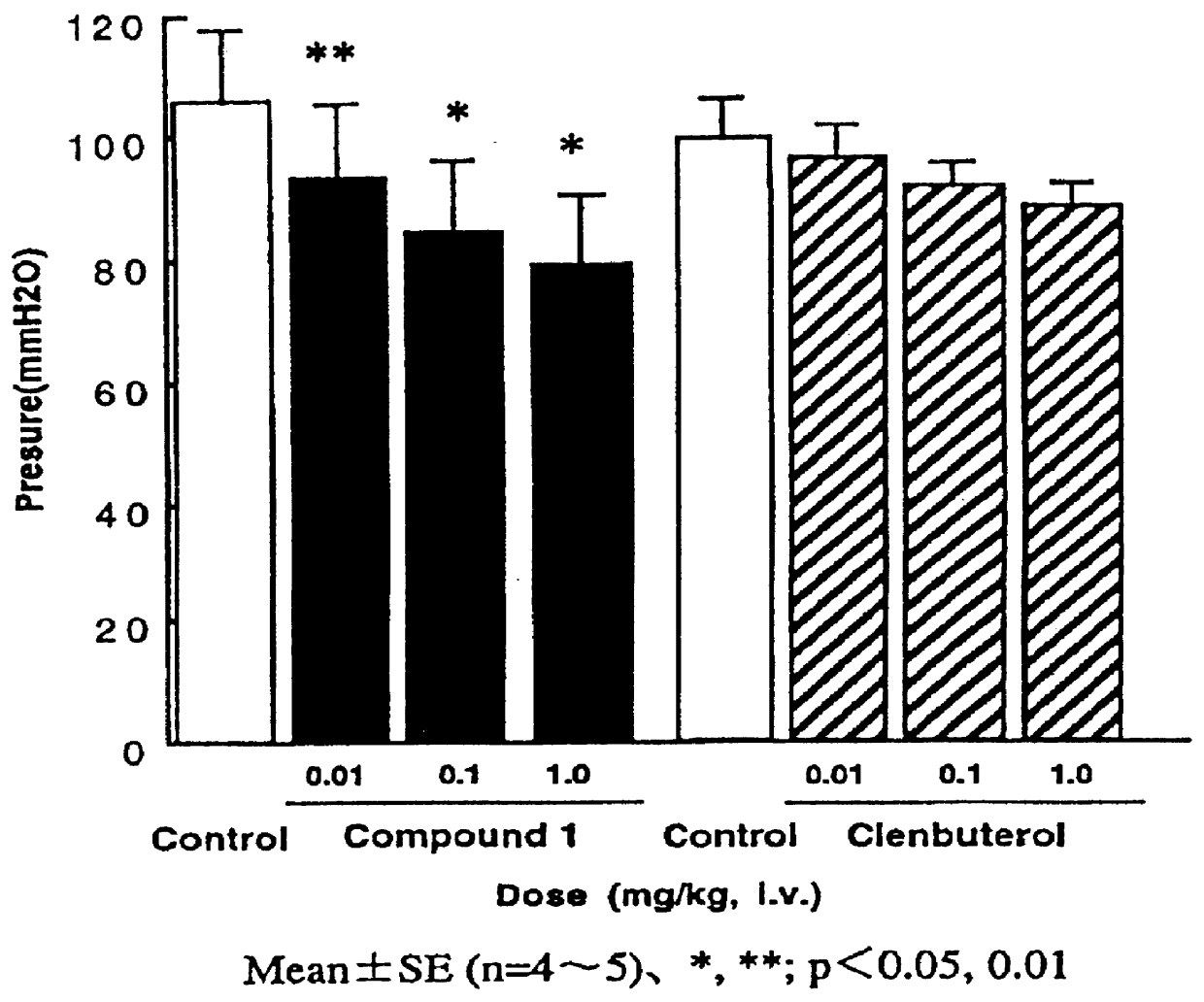
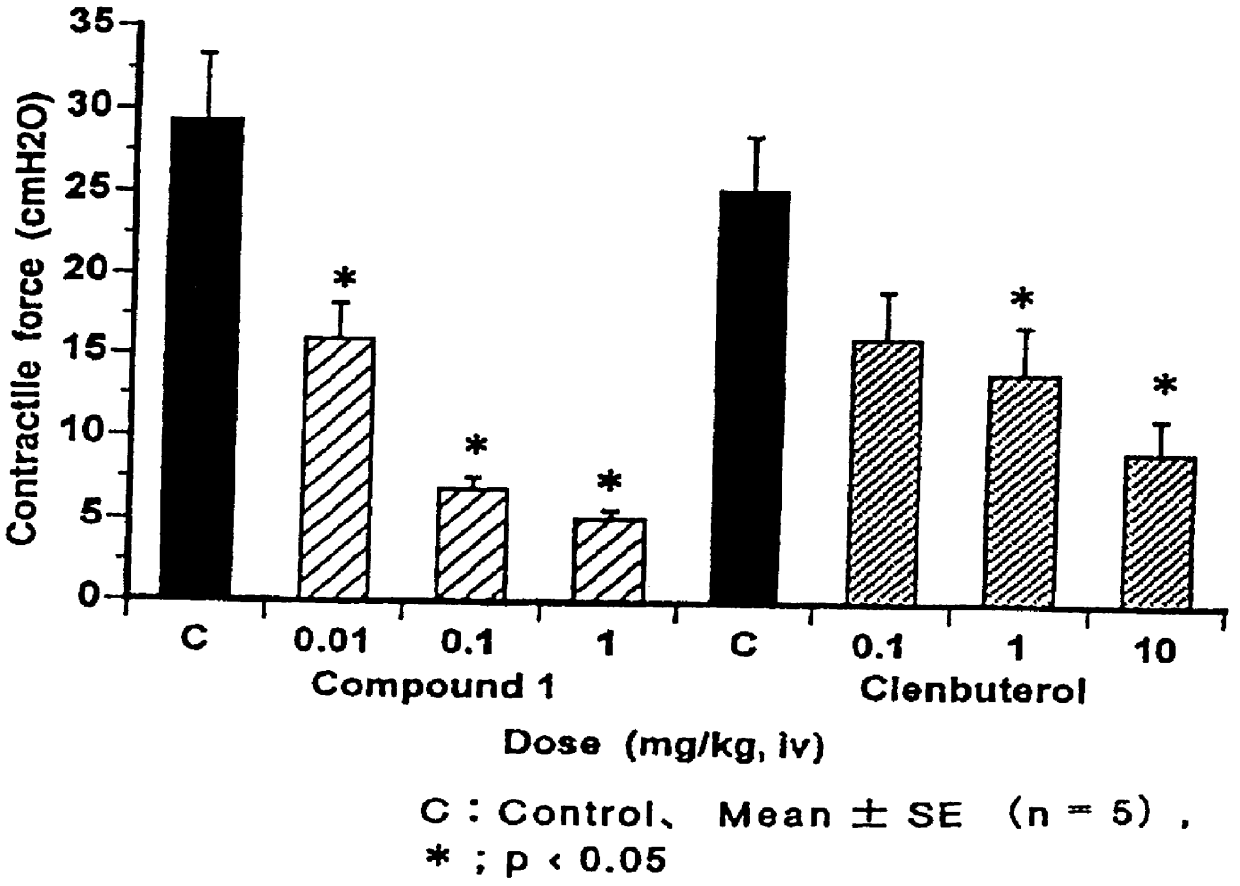
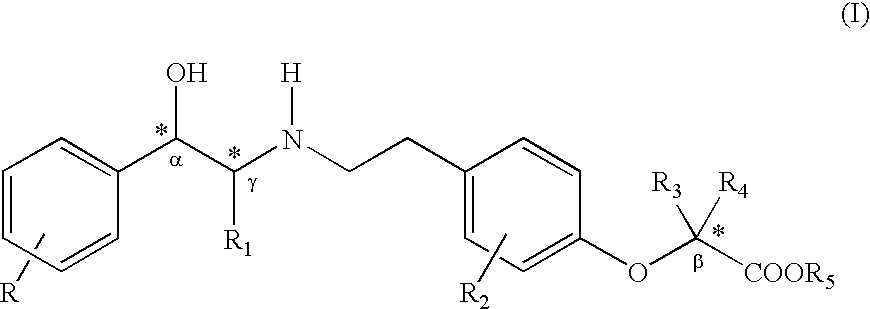
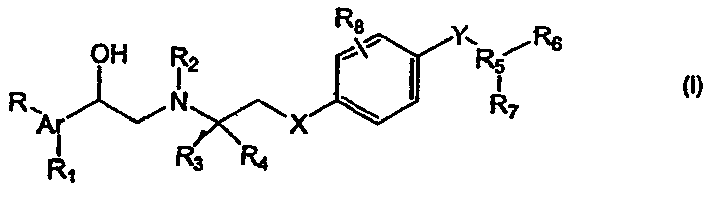
PCT No. PCT / JP96 / 03097 Sec. 371 Date Apr. 24, 1998 Sec. 102(e) Date Apr. 24, 1998 PCT Filed Oct. 24, 1996 PCT Pub. No. WO97 / 15549 PCT Pub. Date May 1, 1997The present invention relates to phenylethanolamine compounds represented by general formula [I]: (where R1 represents hydrogen or halogen; R2 represents hydrogen, hydroxy, lower alkoxy, lower alkoxy substituted with one or two lower alkoxycarbonyl or carboxy groups, lower alkoxy substituted with lower alkylaminocarbonyl which may be substituted with lower alkoxy, lower alkoxy substituted with cyclic aminocarbonyl of 4 to 6 carbon atoms, lower alkoxycarbonyl or carboxy; R3 represents hydrogen, hydroxy, lower alkoxy or lower alkoxy substituted with one or two lower alkoxycarbonyl or carboxy groups; R2 and R3 may be bonded to each other to form methylenedioxy substituted with carboxy or lower alkoxycarbonyl; and m and n are 0 or 1), and their pharmacologically acceptable salts, which have a potent beta 3 adrenergic stimulating effect and high beta 3 adrenergic receptor selectivity, as well as to processes for their production and intermediates in their production.
Owner:TT PHARMA
Beta adrenergic receptor agonists for the treatment of b-cell proliferative disorders
InactiveUS20100009934A1Reduce the impactBiocideHydroxy compound active ingredientsAdrenergicInhalation
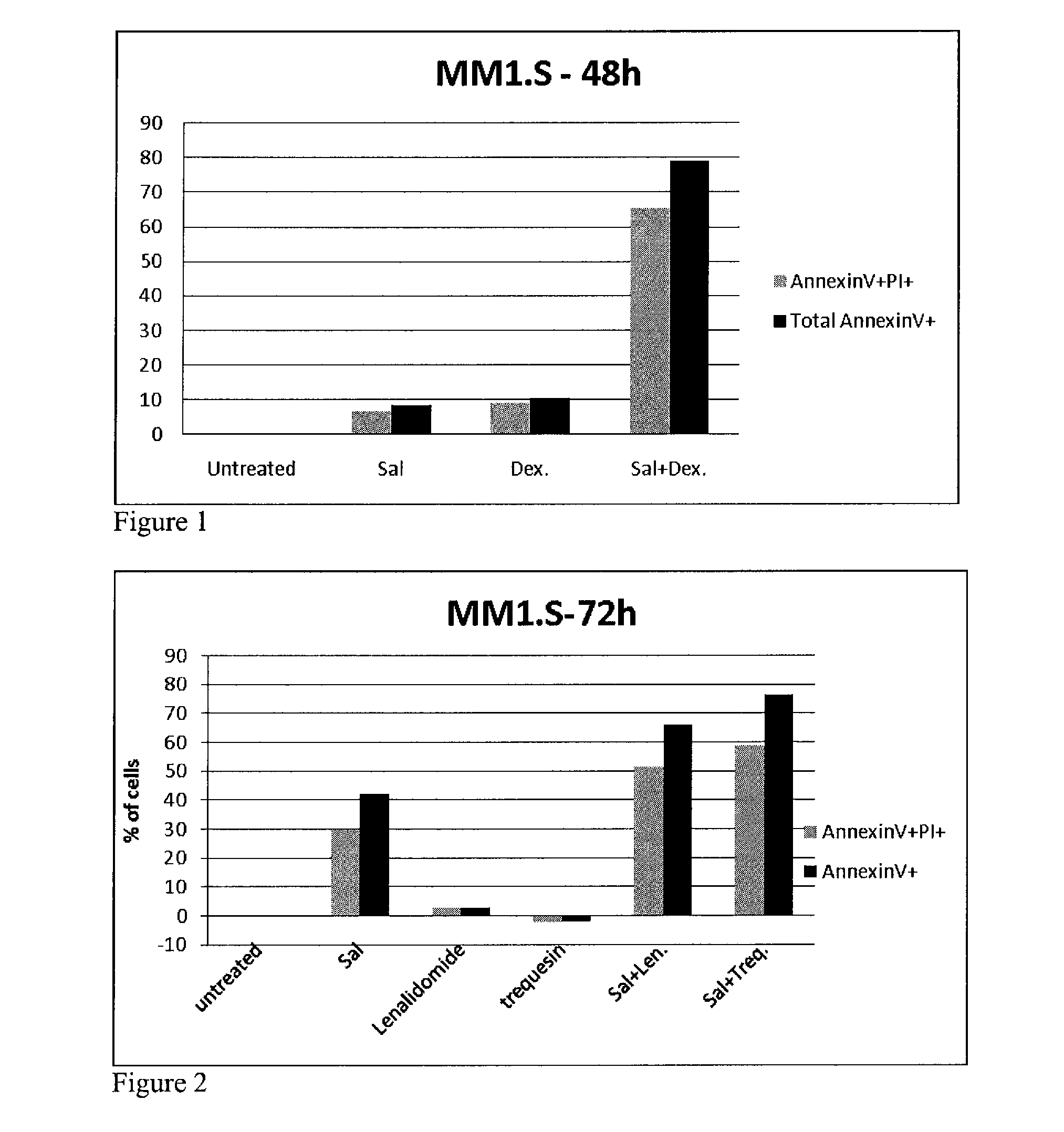
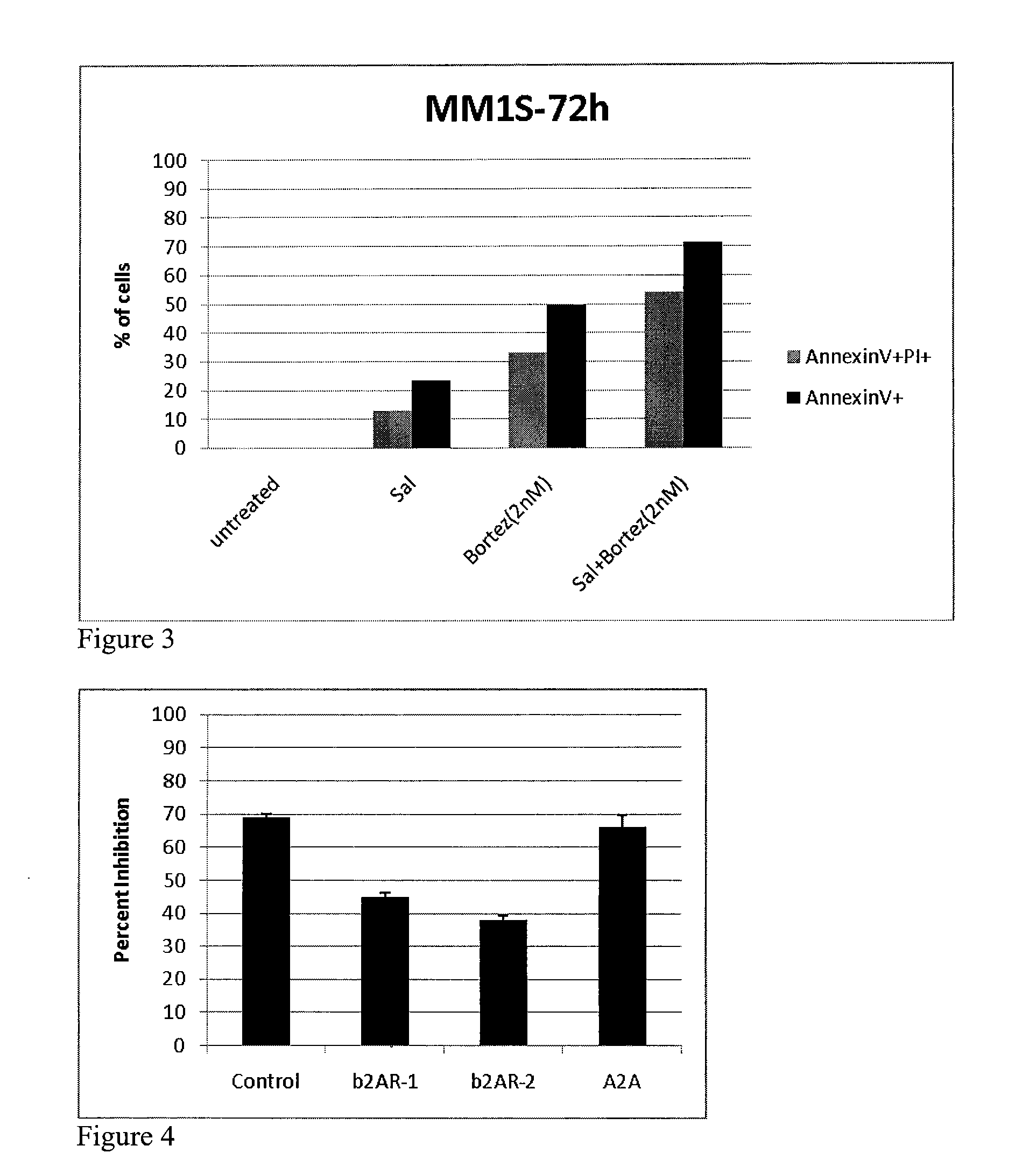
The invention features a method of treating a B-cell proliferative disorder by administering to a patient a BAR agonist, e.g., formulated for administration by a route other than inhalation (such as for oral or intravenous administration), in an amount effective to treat the B-cell proliferative disorder. The BAR agonist may be administered as a monotherapy or in combination with one or more other agents, e.g., a PDE inhibitor, an A2A receptor agonist, or an antiproliferative compound, in amounts that together are effective to treat the B-cell proliferative disorder. The invention further features pharmaceutical compositions and kits including a BAR agonist, alone or in combination with additional agents, for the treatment of a B-cell proliferative disorder.
Owner:ZALICUS INC
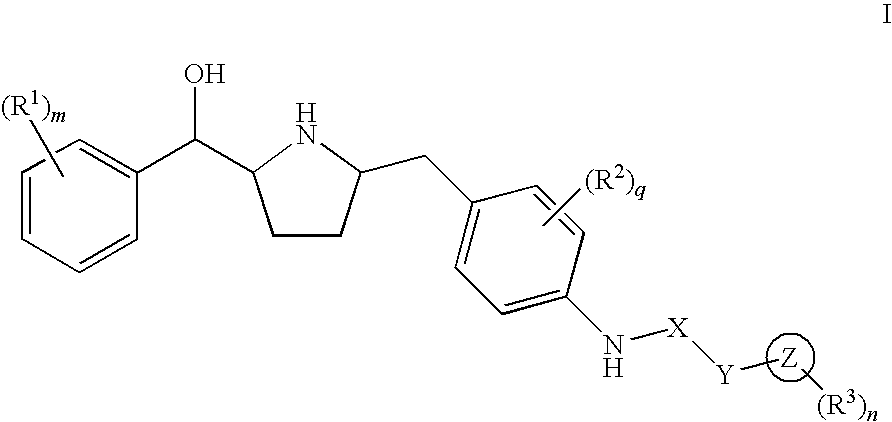
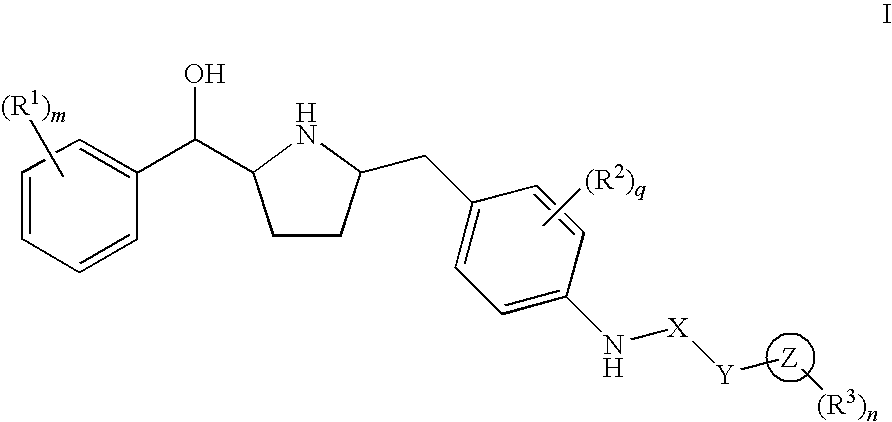
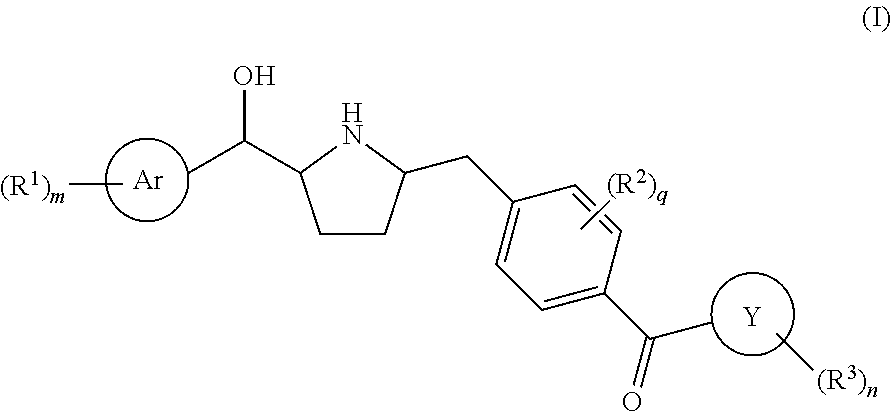
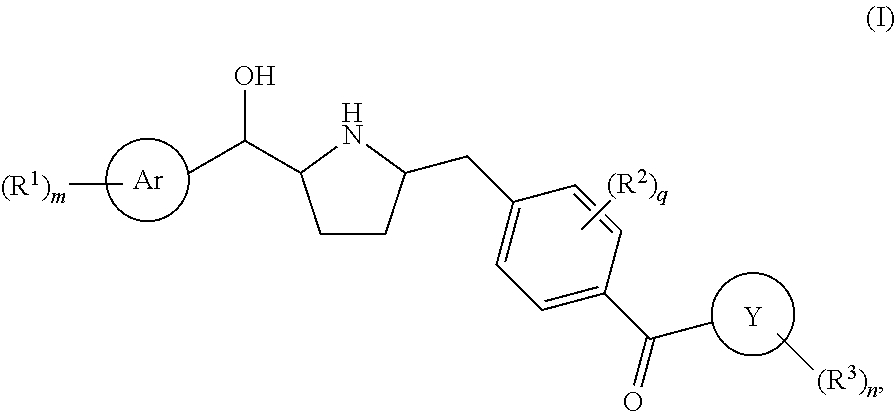
Hydroxymethyl pyrrolidines as beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME CORP
Pharmaceutical combination
ActiveUS20130172277A1High potencyImprove efficacyBiocidePharmaceutical delivery mechanismMuscarinic antagonistAdrenergic
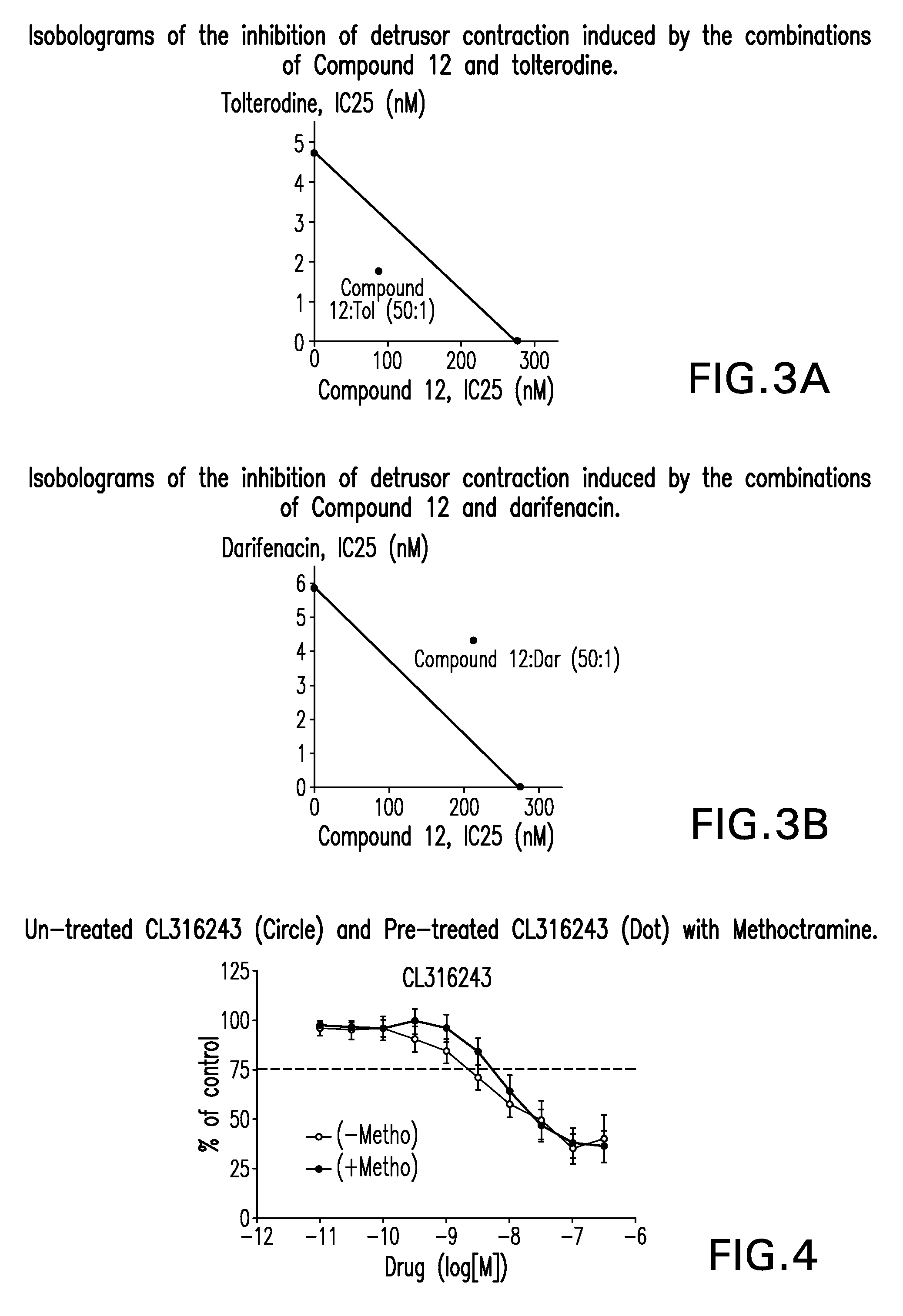
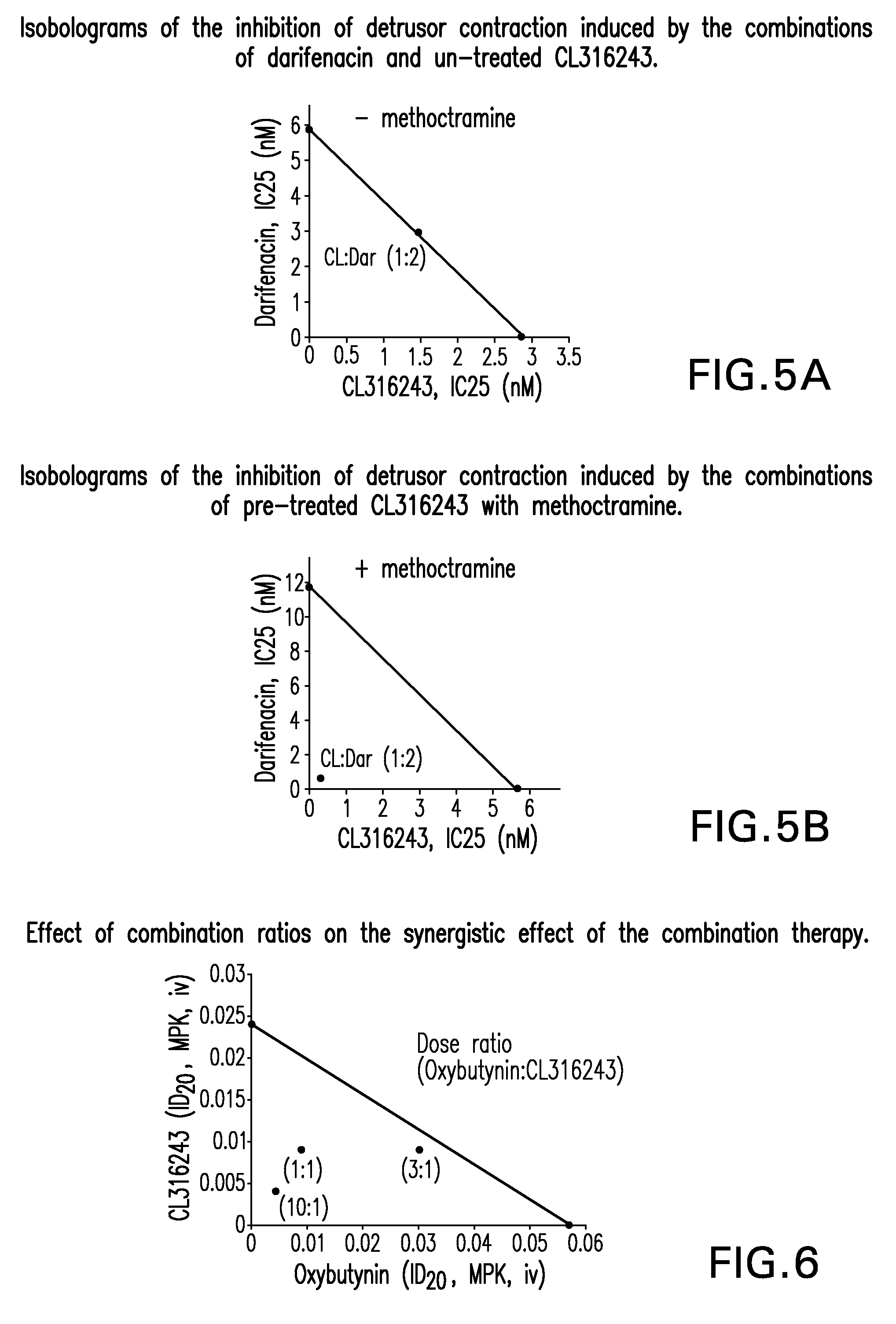
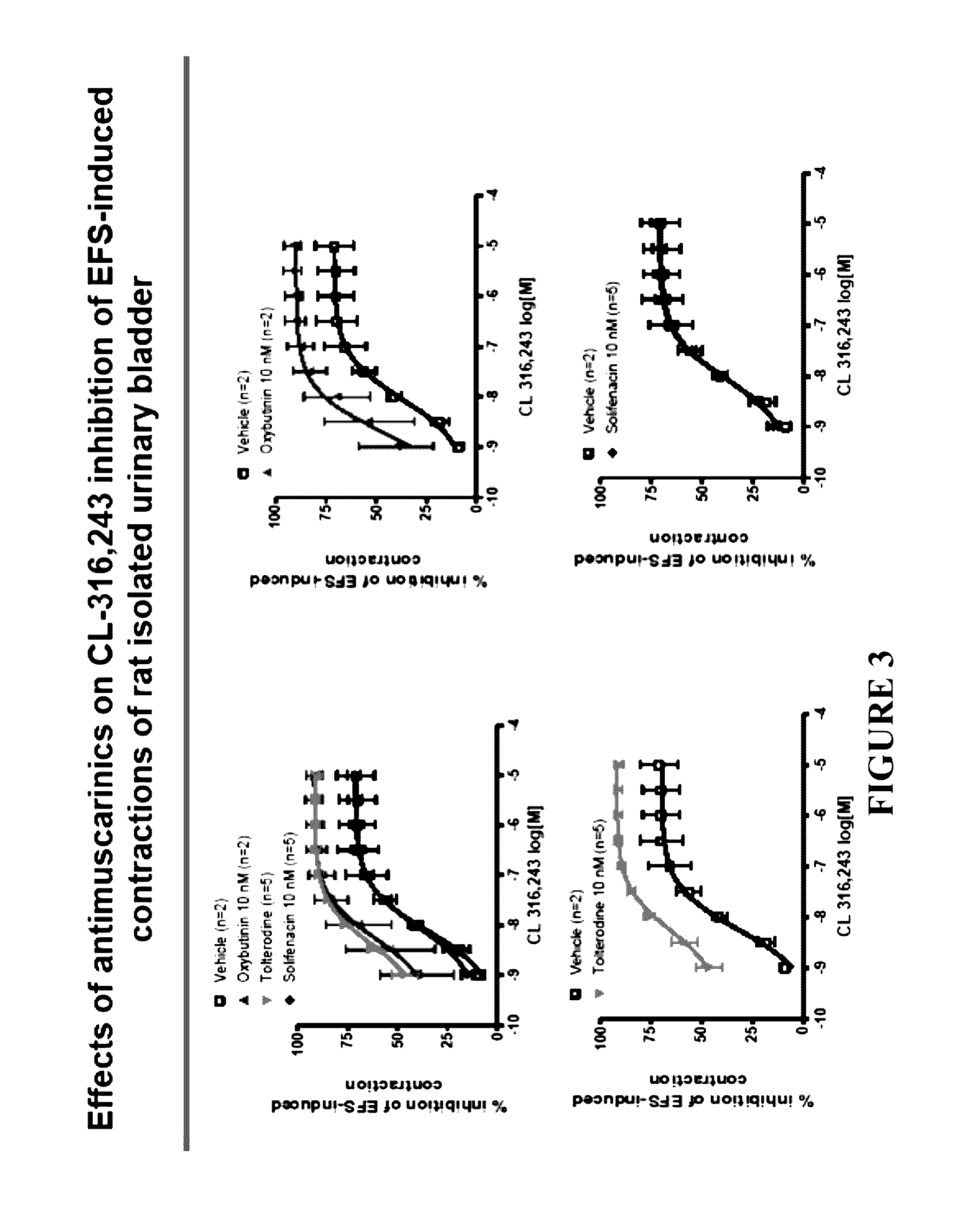
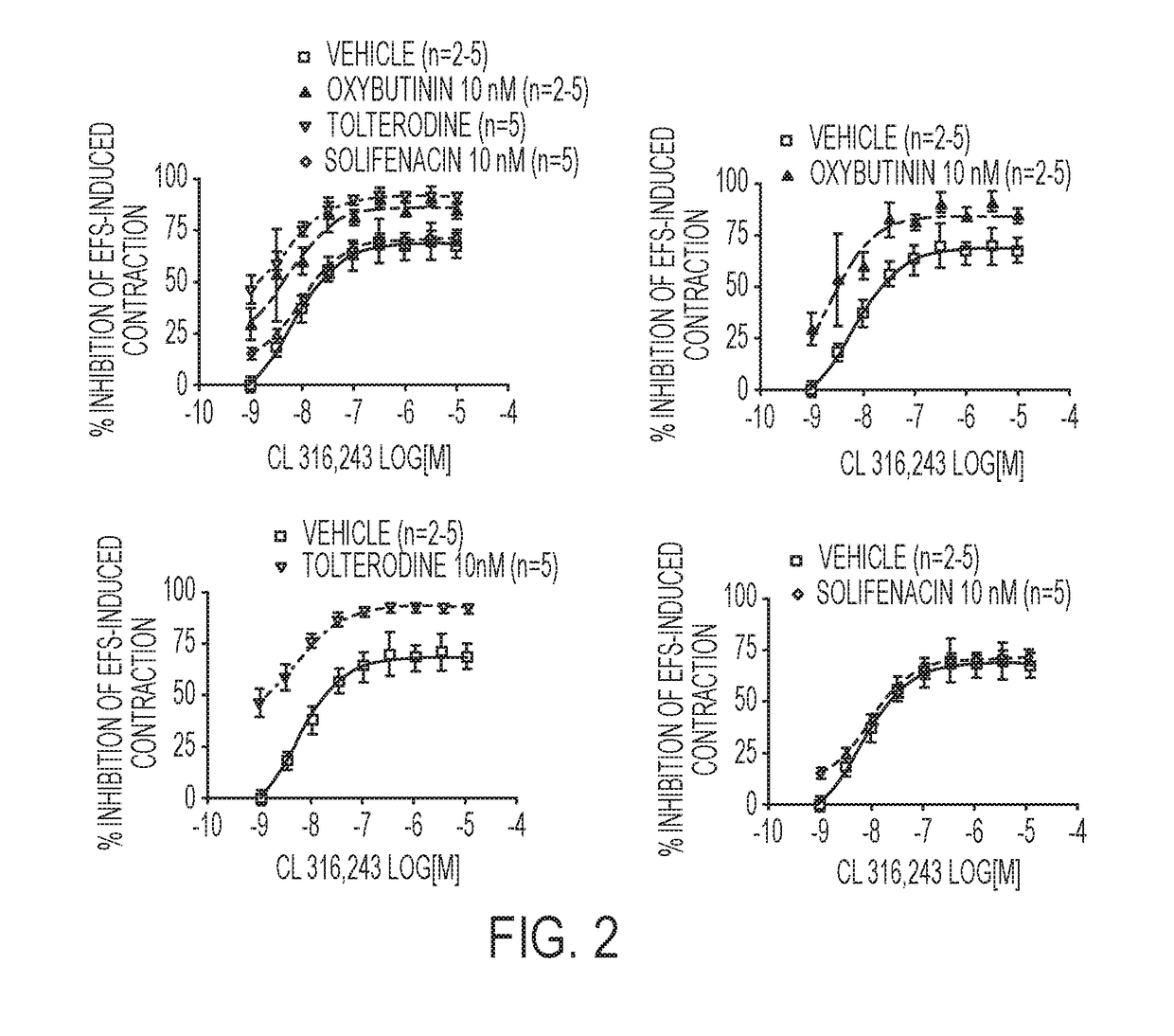
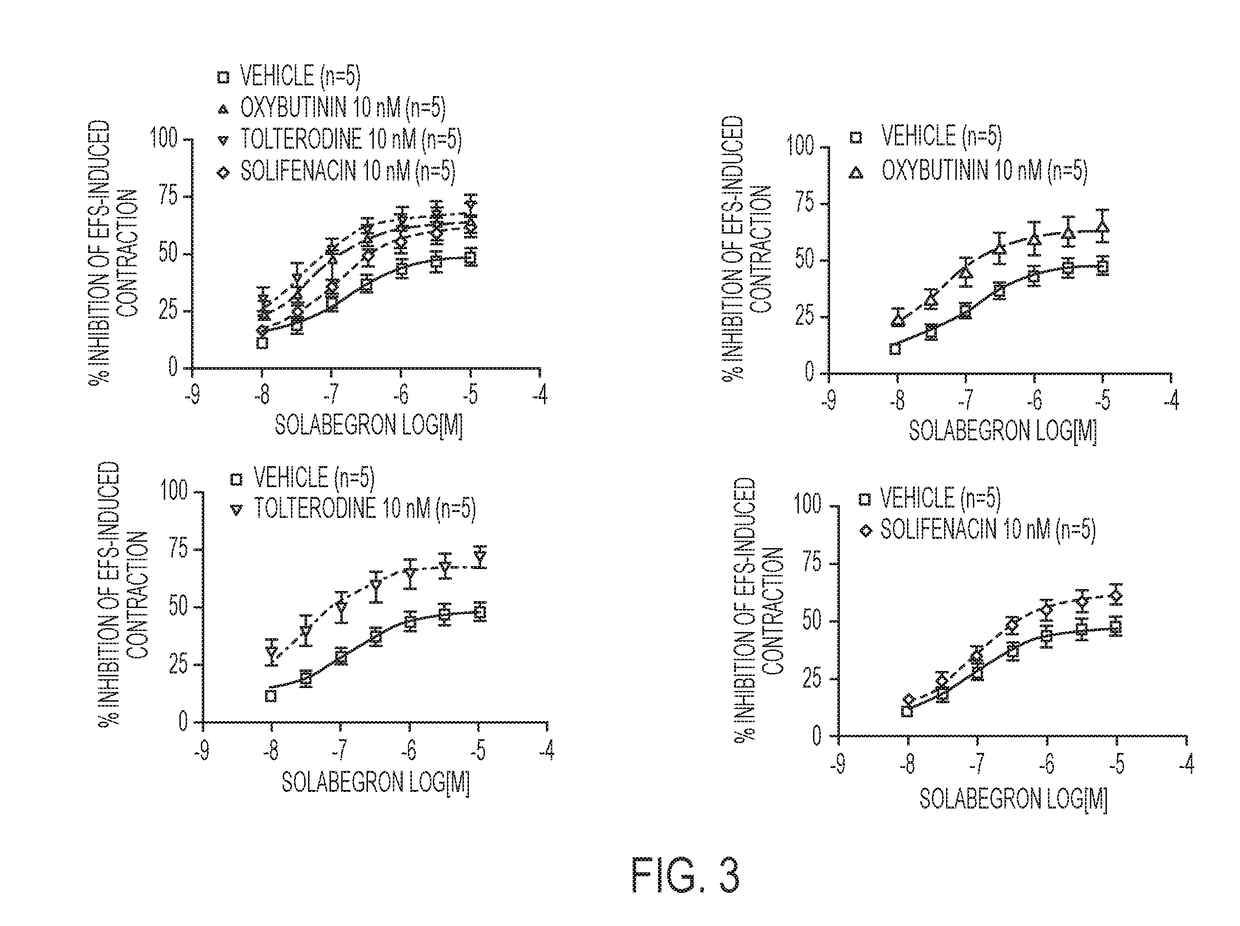
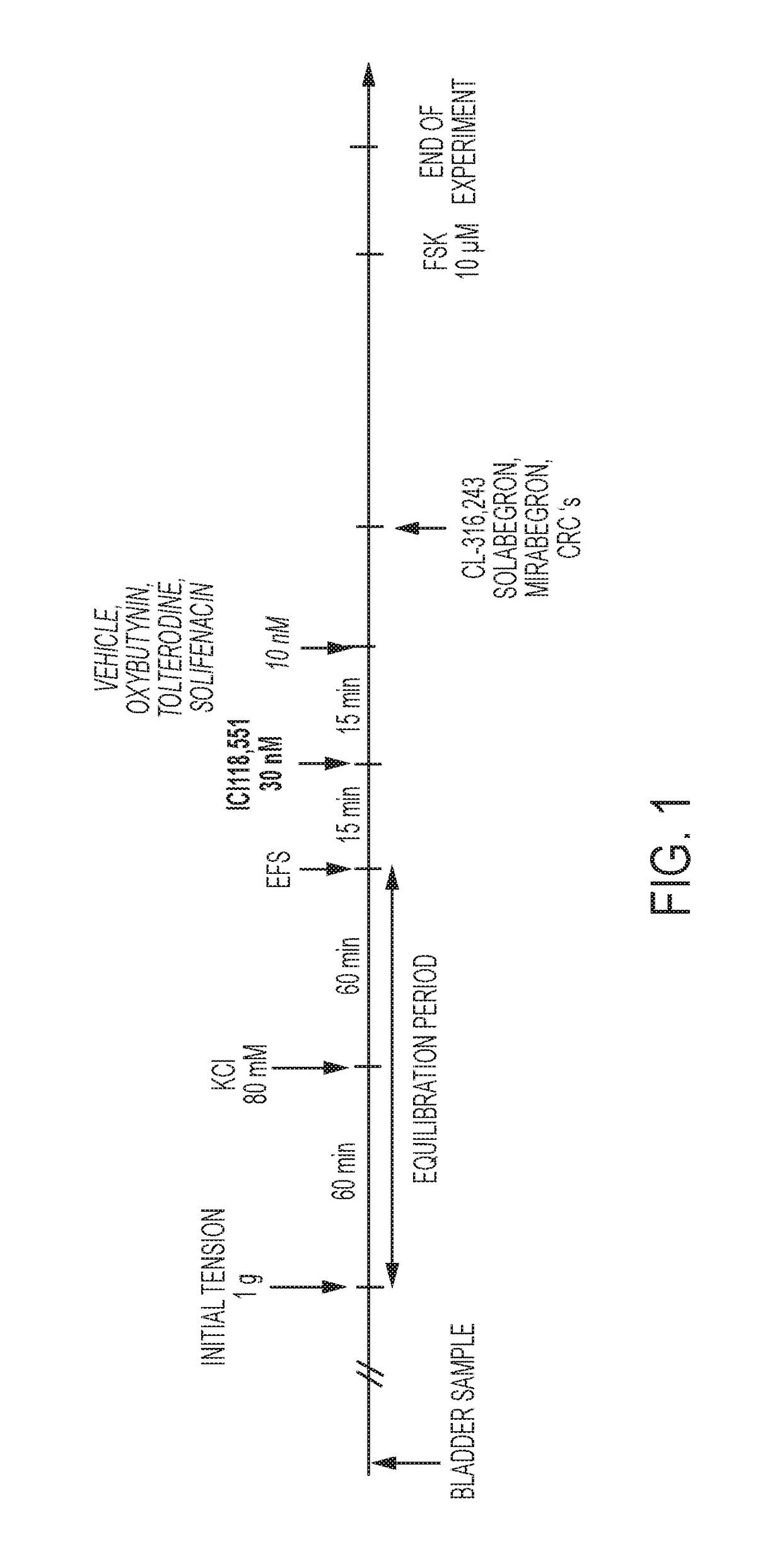
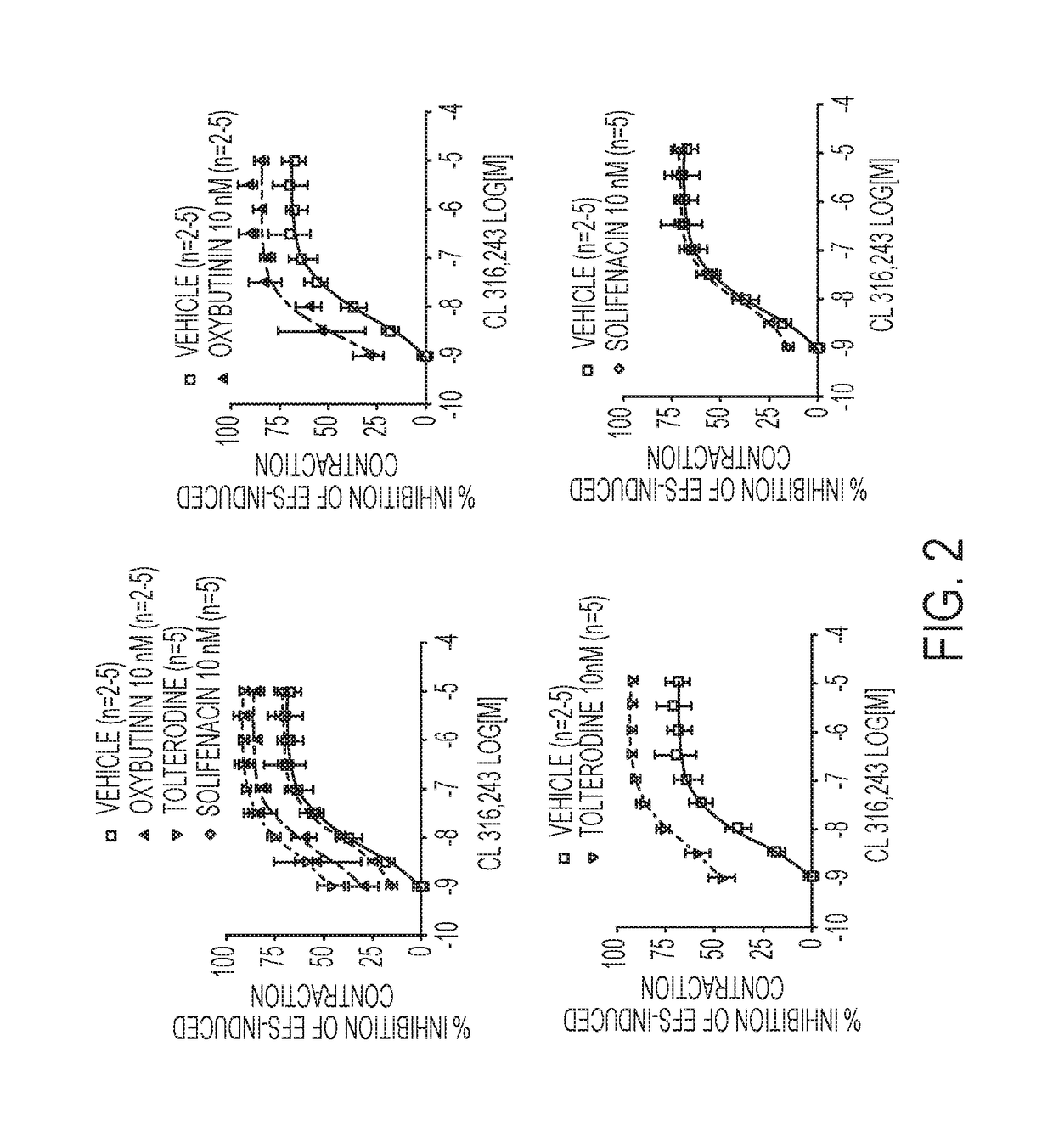
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
Combination therapy using a beta 3 adrenergic receptor agonists and an antimuscarinic agent
Described herein is an improved method of treating overactive bladder, wherein the method comprises administering to a patient in need thereof a beta 3 adrenergic receptor agonist, an antimuscarinic agent, and an optional selective M2 antagonist. Such combination therapy provides improved efficacy and / or reduced side effects.
Owner:MERCK SHARP & DOHME CORP
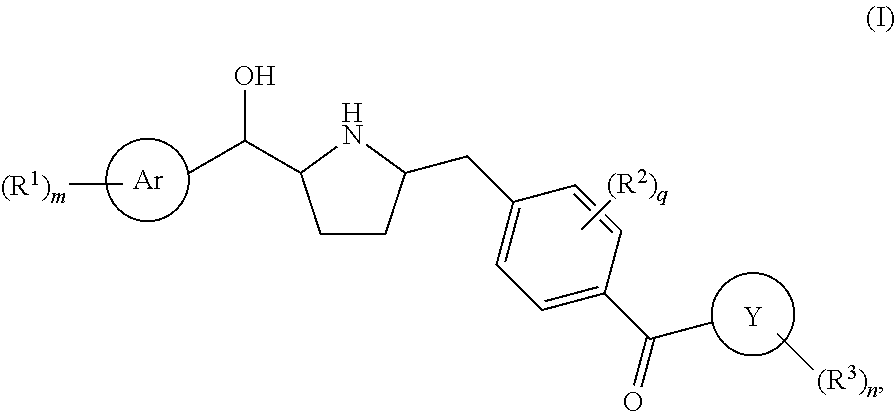
Hydroxymethyl pyrrolidines as beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME CORP
Carbazolyl-substituted ethanolamines as selective beta -3 agonists
InactiveUS6140352AAvoid insufficient purityBiocideNervous disorderAdrenergic receptor agonistsEthanolamines
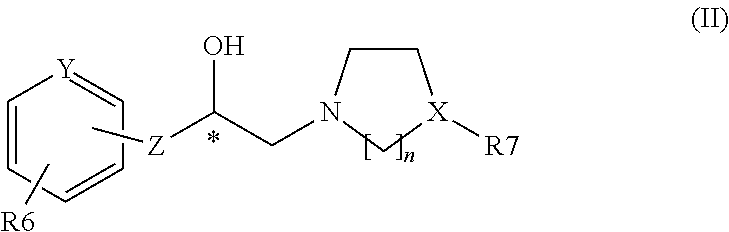
PCT No. PCT / US97 / 15230 Sec. 371 Date May 4, 1998 Sec. 102(e) Date May 4, 1998 PCT Filed Aug. 28, 1997 PCT Pub. No. WO98 / 09625 PCT Pub. Date Mar. 12, 1998Disclosed herein are selective beta 3 adrenergic agonists represented by the following structural formula: The variables in the structural formula shown above are defined in the specification. Also disclosed are methods of using these compounds for agonizing the beta 3 adrenergic receptor in patients in need of such treatment, for example, patients in need of treatment for obesity or Type II diabetes.
Owner:ELI LILLY & CO
Novel beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME LLC
Pharmaceutical combinations
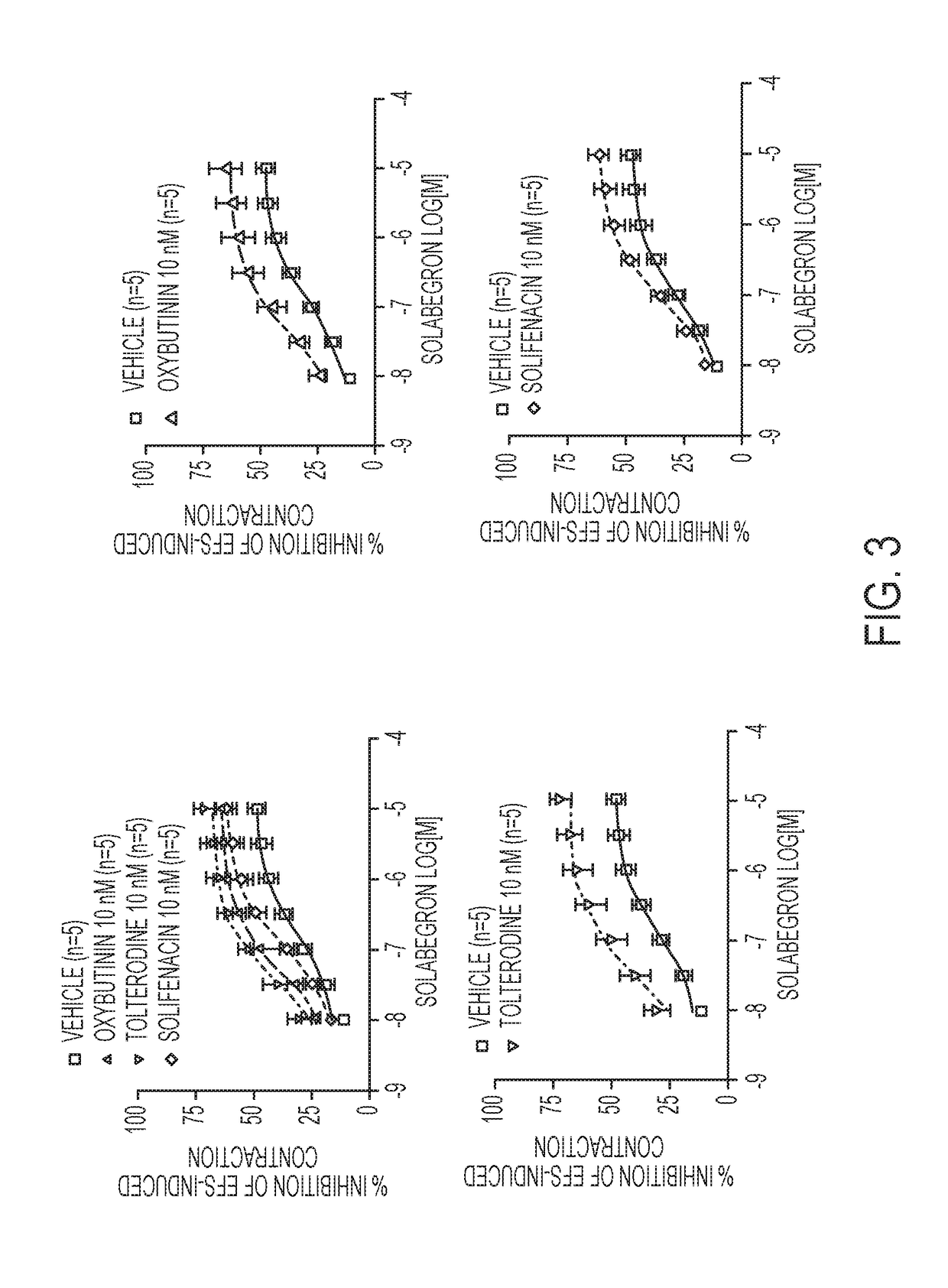
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Disclosed combinations include solabegron and oxybutynin. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
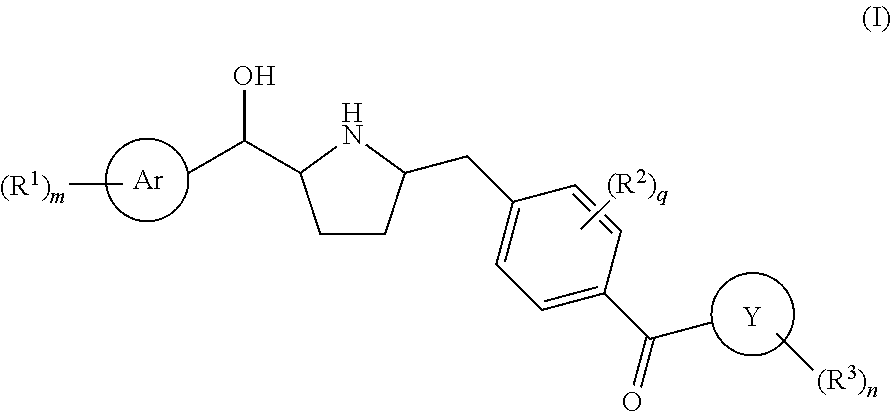
Novel pyrrolidine derived beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME LLC
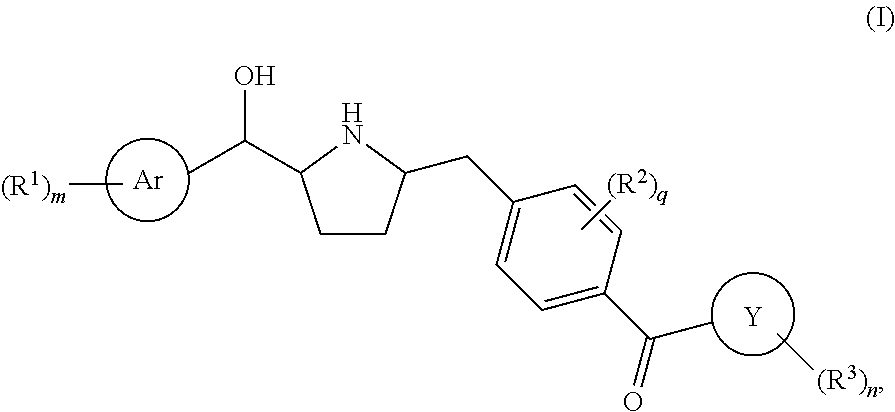
Pyrrolidine derived beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME LLC
Hydroxymethyl pyrrolidines as beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME LLC
Novel pyrrolidine derived beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME LLC
Cyclic amine phenyl beta-3 adrenergic receptor agonists
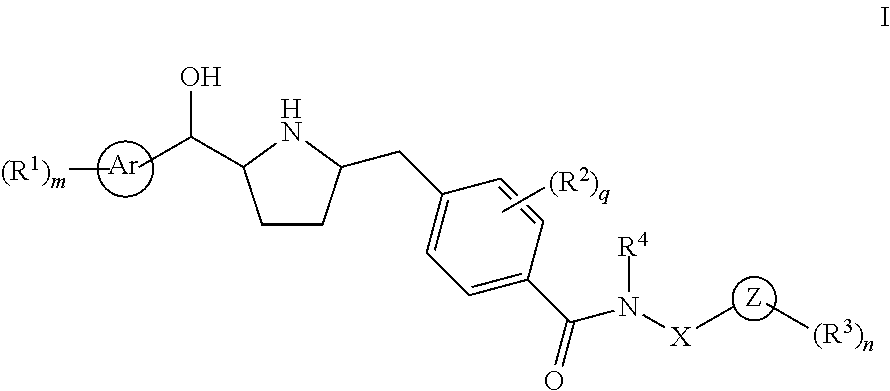
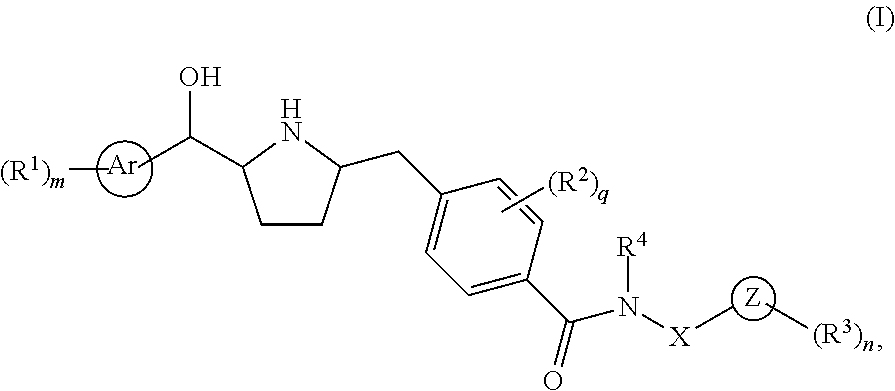
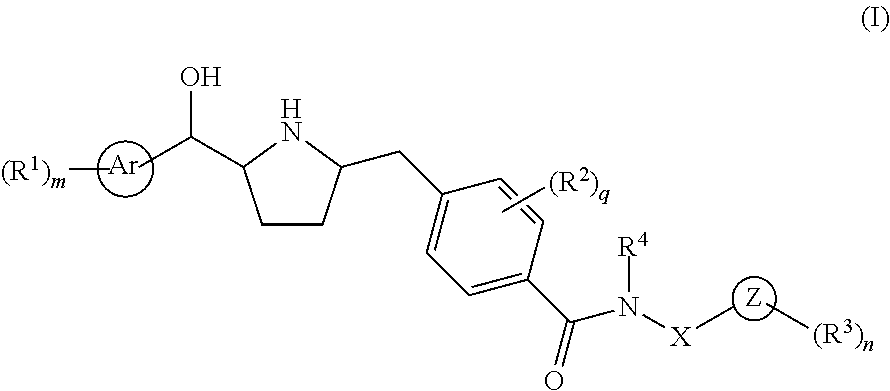
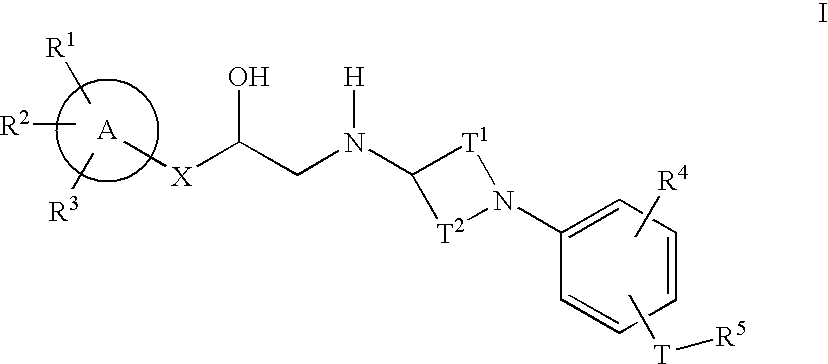
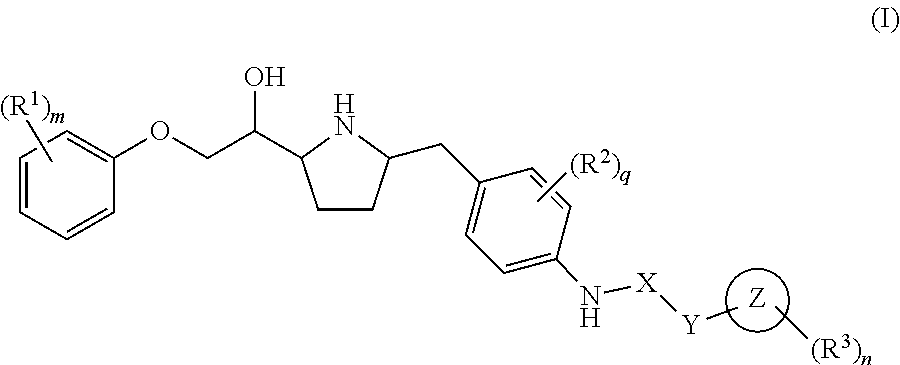
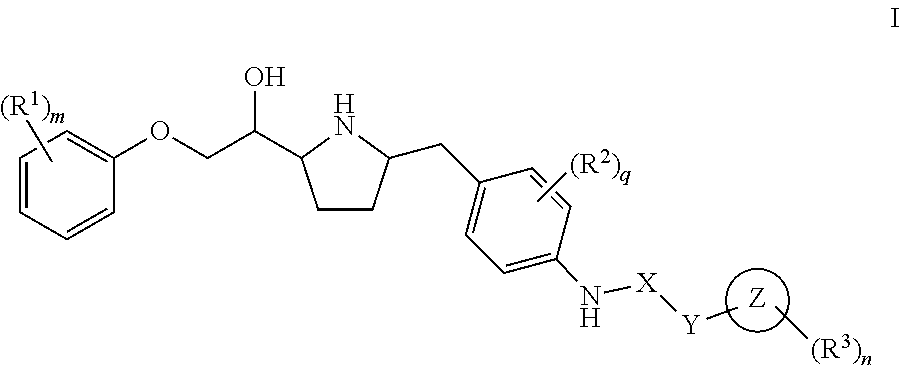
This invention provides compounds of Formula I having the structure wherein,R1, R2, R3, R4, R5, T, T1, T2, and X are as defined hereinbefore, or a pharmaceutically acceptable salt thereof, which are useful in treating or inhibiting metabolic disorders related to insulin resistance or hyperglycemia (typically associated with obesity or glucose intolerance), atherosclerosis, gastrointestinal disorders, neurogenetic inflammation, glaucoma, ocular hypertension and frequent urination; and are particularly useful in the treatment or inhibition of type II diabetes.
Owner:WYETH LLC
Pharmaceutical Combination
Pharmaceutical combinations comprising a beta-3 adrenergic receptor agonist and a muscarinic receptor antagonist, and methods for their use are disclosed. Methods of using the pharmaceutical combinations for the treatment of one or more symptoms associated with overactive bladder, for example, frequency of urgency, nocturia, and urinary incontinence, are also disclosed.
Owner:B3AR THERAPEUTICS INC
Beta-3 receptor ligands and their use in therapy
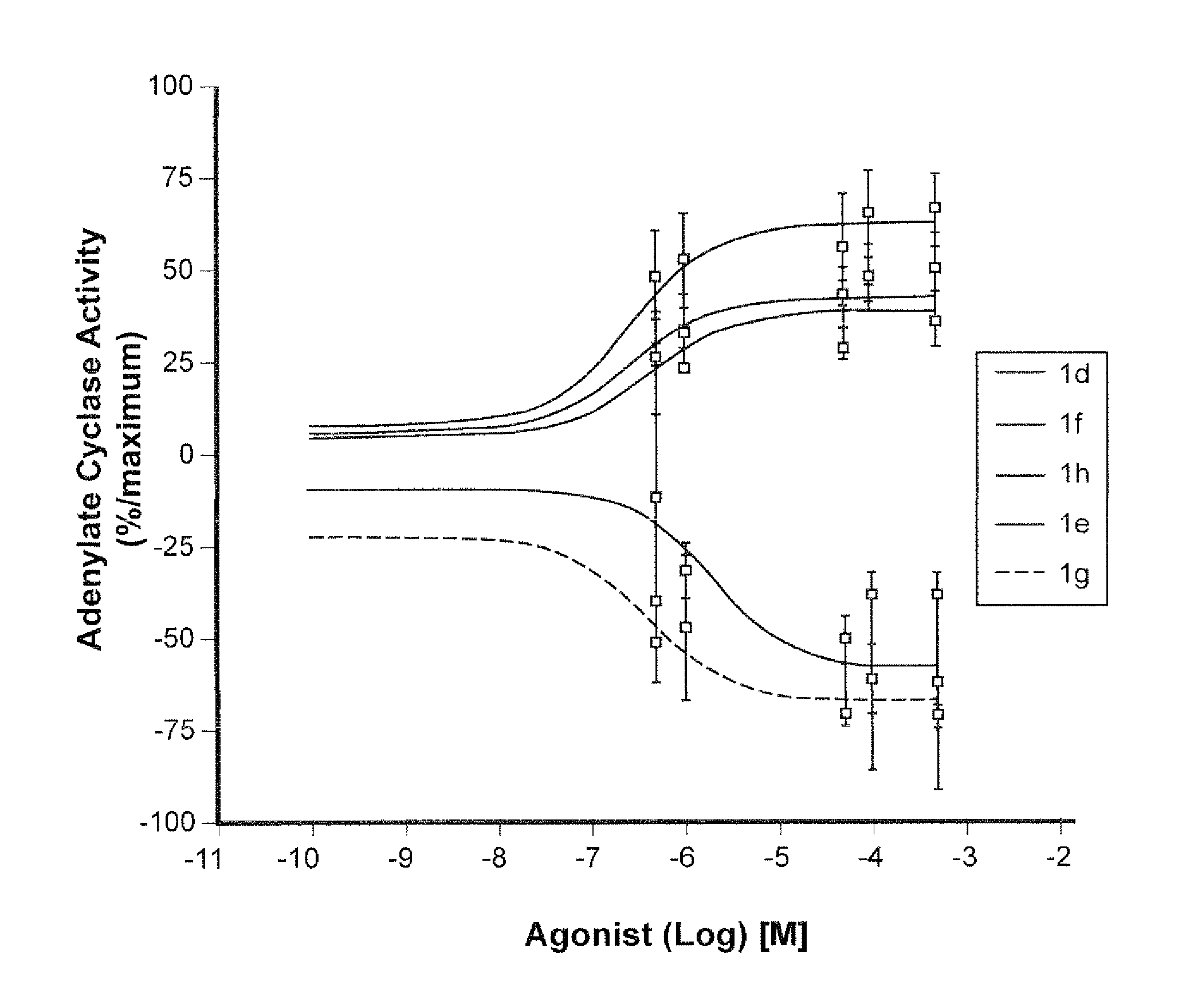
The present invention relates to new compounds, ligands of the beta-3 adrenergic receptor, their preparation and their use in therapy or as research tools for said receptor; the invention also relates to a process for the preparation of the compounds of the invention and the use of inverse agonists of the beta-3 adrenergic receptor as medicaments.
Owner:UNVERSITA DEGLI STUDI DI BARI
Pyrrolidine-derived beta 3 adrenergic receptor agonists
Owner:MERCK SHARP & DOHME LLC
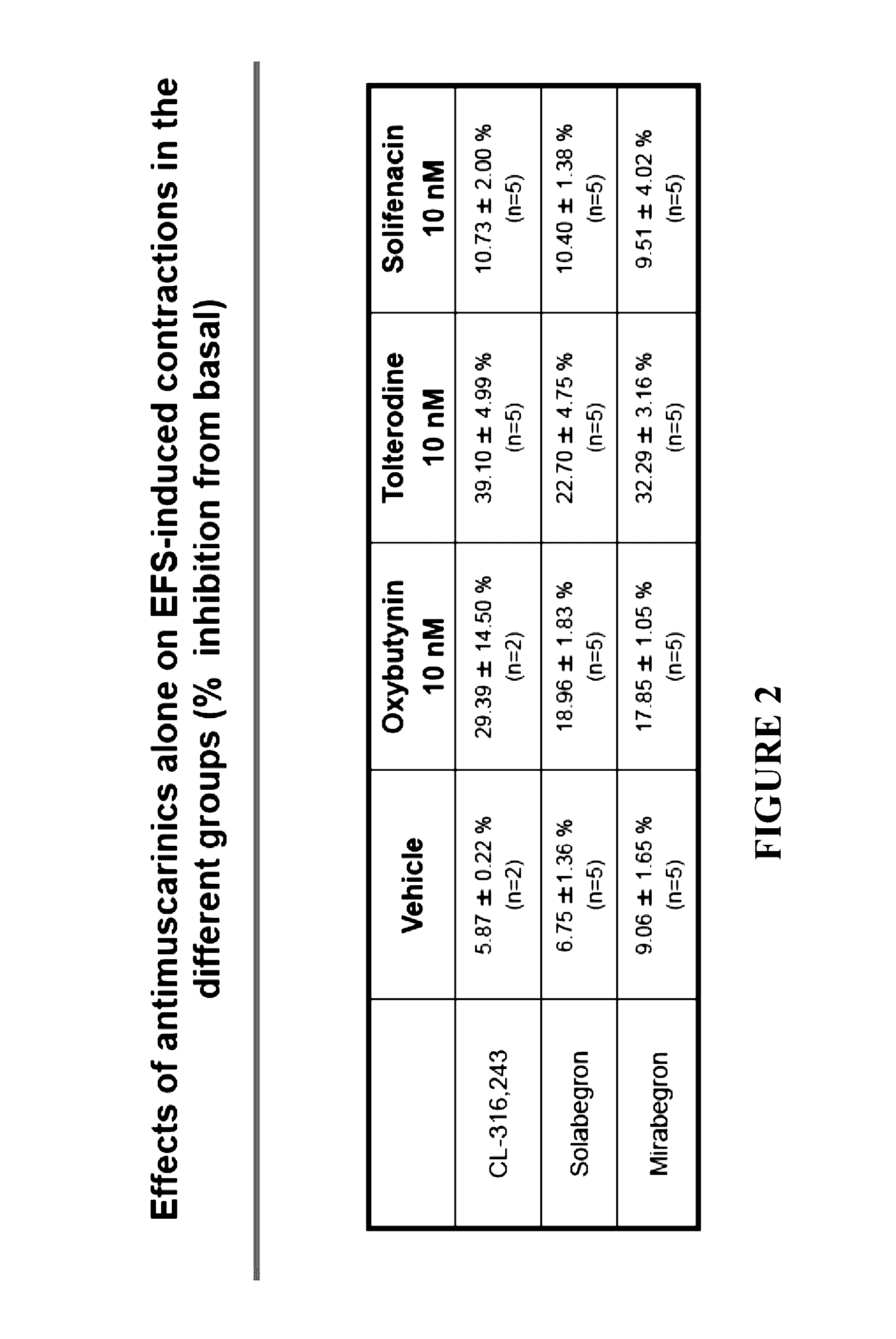
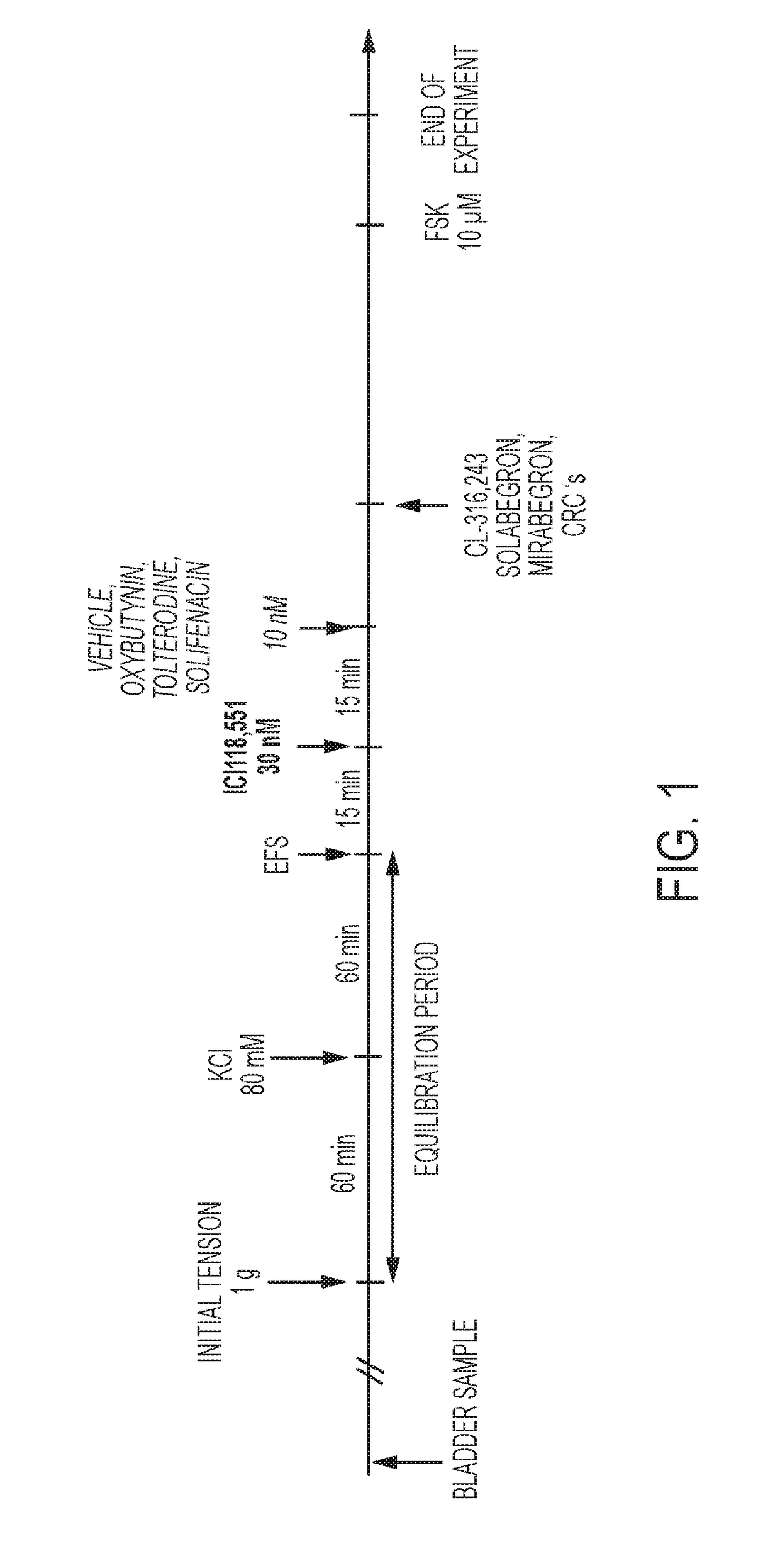
Pharmaceutical compositions and the treatment of overactive bladder
ActiveUS20170151199A1Decrease micturition frequencyOrganic active ingredientsAdrenergicTreatments for overactive bladder
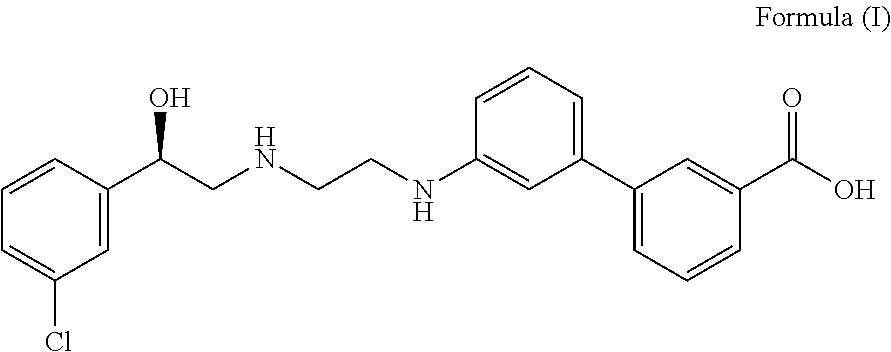
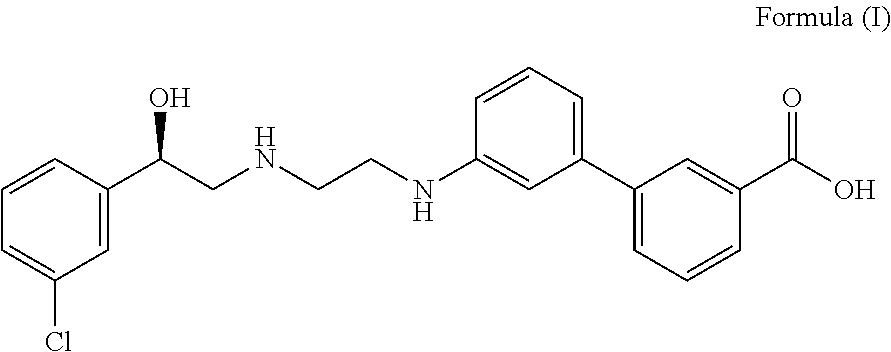
The present invention relates to methods of treating overactive bladder and the symptoms associated therewith, for example, urinary urgency, frequency of mictruitions, nocturia, and urgency urinary incontinence. One treatment method according to the present invention comprises treatment with the beta-3 adrenergic receptor agonist solabegron. Another treatment combination according to the invention comprises solabegron, and a muscarinic receptor antagonist which results in a synergistic effect on the symptoms associated with OAB.
Owner:B3AR THERAPEUTICS INC
Drug response marker in beta-1 adrenergic receptor gene
InactiveUS7195873B2Respond effectivelyMicrobiological testing/measurementReceptors for neuromediatorsAdrenergicTrial drug
Methods of using a genetic polymorphic variation in the human beta-1 adrenergic receptor gene as a drug response marker are presented. Determining the presence or absence of the A145G genetic variation in the human beta-1 adrenergic receptor gene is useful in predicting an individual's relative response to different antihypertensive drugs; optimizing antihypertensive treatment for an individual; selecting candidate human subjects for participation in clinical trials involving antihypertensive drugs; and, predicting the relative responses among a plurality of individuals to an antihypertensive drug.
Owner:MYRIAD GENETICS
Pharmaceutical compositions and the treatment of overactive bladder
The present invention relates to methods of treating overactive bladder and the symptoms associated therewith, for example, urinary urgency, frequency of mictruitions, nocturia, and urgency urinary incontinence. One treatment method according to the present invention comprises treatment with the beta-3 adrenergic receptor agonist solabegron. Another treatment combination according to the invention comprises solabegron, and a muscarinic receptor antagonist which results in a synergistic effect on the symptoms associated with OAB.
Owner:B3AR THERAPEUTICS INC
Agents derived from holoptelea integrifolia and their compositions for the control of metabolic syndrome and associated diseases
ActiveUS20120231095A1BiocideHydroxy compound active ingredientsDiseaseAdipose Differentiation-Related Protein
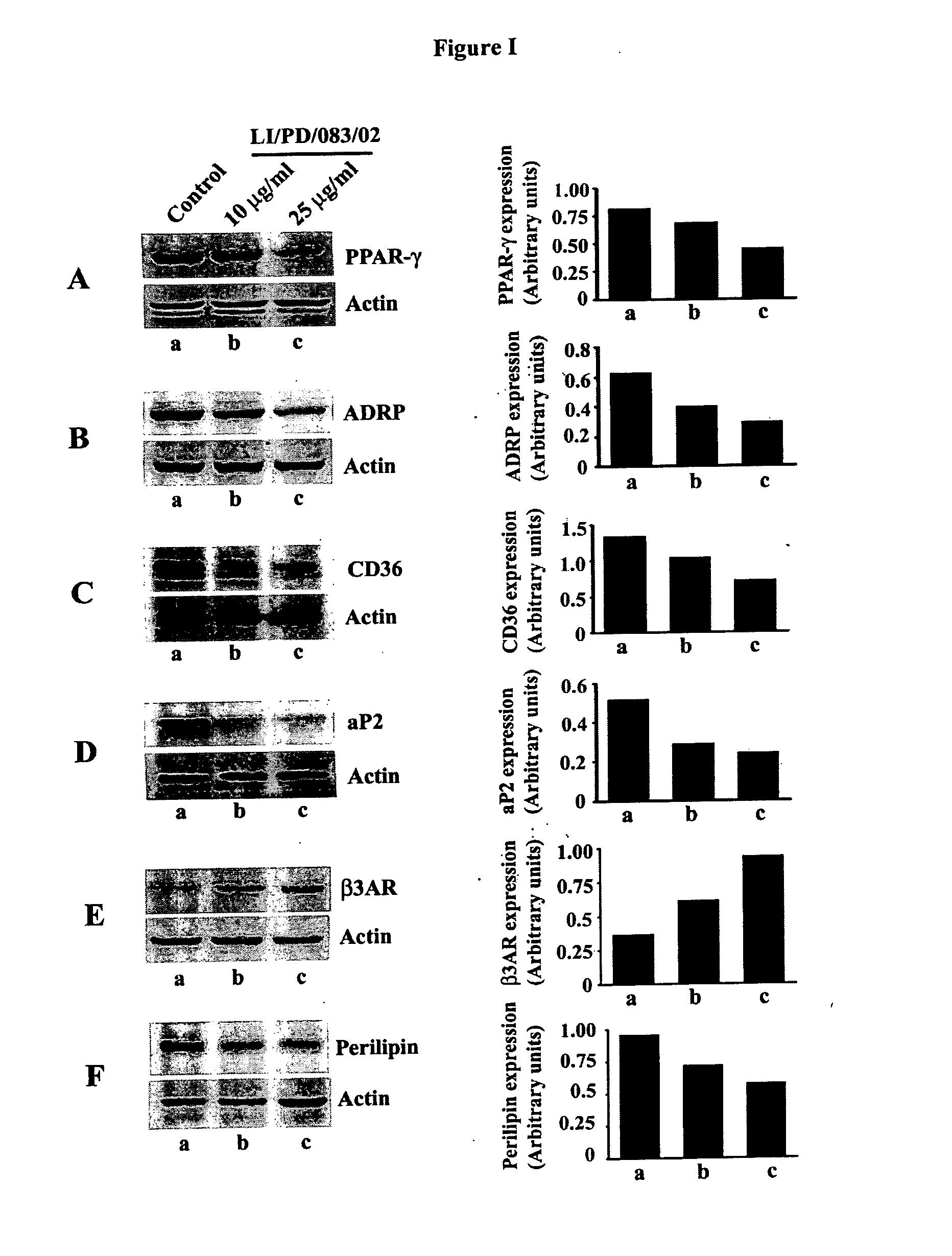
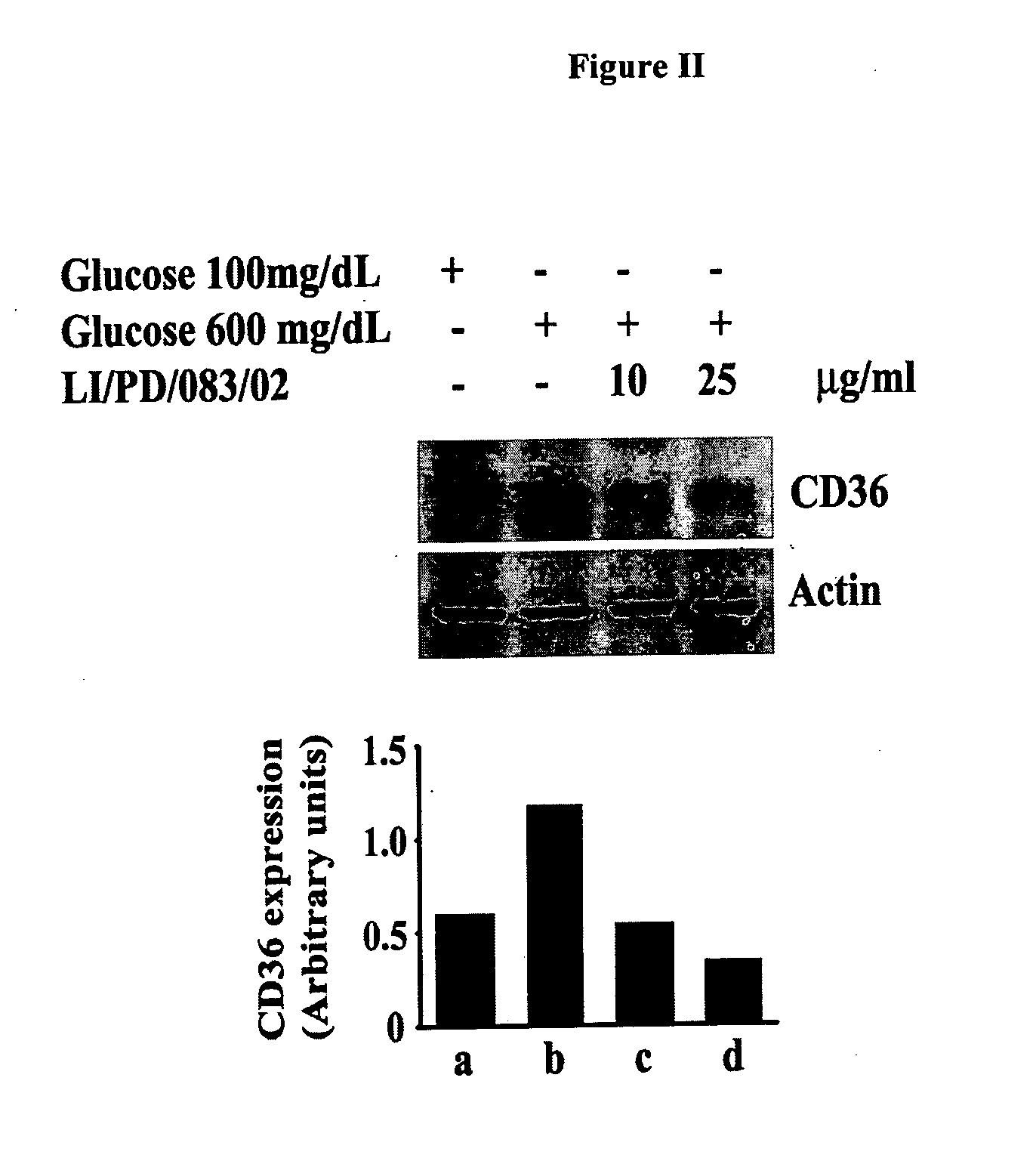
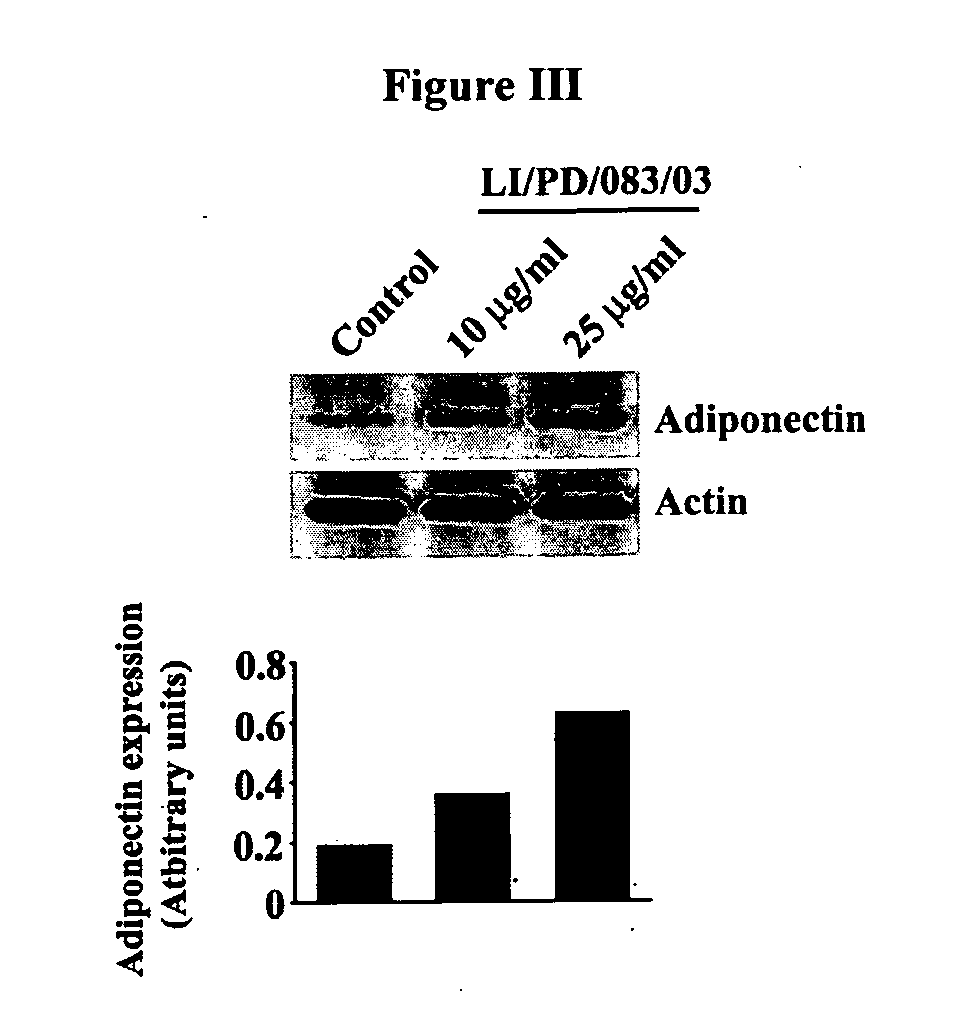
The invention discloses phytochemical agents derived from Holoptelea integrifolia and novel composition(s) comprising at least one component selected from the extract(s), fraction(s) and active compound(s) for the protection and alleviation of Metabolic Syndrome, insulin resistance, endothelial dysfunction, chronic kidney disease, atherosclerosis, diabetes and other disease conditions associated with metabolic syndrome. The invention also discloses the amelioration of certain biomarker molecules such as Peroxisome proliferator-activated receptor gamma (PPAR-γ), Adipose Differentiation Related Protein (ADRP), CD36, Adipocyte Fatty-acid-Binding Protein (aP2 / FABP-4 / A-FABP), beta-3 Adrenergic Receptor (β3AR), Leptin, Perilipin and Adiponectin by using the phytochemical components derived from Holoptelea integrifolia.
Owner:LAILA NUTRACEUTICALS
Method for assessing lipid metabolism type obese gene constitution
InactiveCN103834739AImprove detection accuracyGood repeatabilityMicrobiological testing/measurementATP-Binding Cassette Transporter A1Biology
The invention discloses a method for assessing a lipid metabolism type obese gene constitution. The method is characterized in that SNPs (Single Nucleotide Polymorphisms) site gene genotype of ABCA1 (ATP binding cassette transporter A1) gene, LPL (Lipoprotein Lipase) gene, PPARG (Peroxisome Proliferator-Activated Receptor Gamma) gene, and ADRB3 (beta 3-Adrenergic receptor) gene of individuals are detected and analyzed at the same time so as to provide assessment on lipid metabolism type obese gene constitution for all detected people. According to the method, the detected people can know own gene constitution condition and understand the fat potential causes before slimming by diet and sports, so as to select the optimal individual nutritional and sports slimming modes and break each obese gene expressive form, and as a result, the most effective and healthy weight losing effect can be realized.
Owner:上海中和堂门诊部有限公司
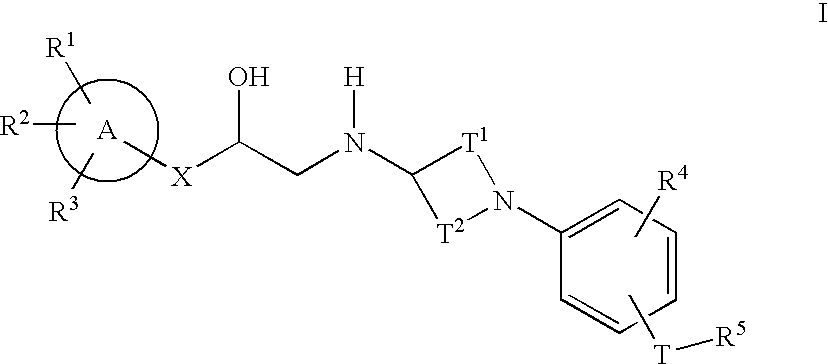
Alpha-aryl ethanolamines and their use as beta-3 adrenergic receptor agonists
The present invention provides β shown in formula (I) 3 Adrenergic receptor agonists, wherein Ar is pyridyl, oxazolyl, thiazolyl or phenyl; R 5 is a 5- or 6-membered heterocycle; X is a bond or oxygen; and Y is a bond, alkylene, OCH 2 、CH 2 O or oxygen; stereoisomers and prodrugs thereof, and pharmaceutically acceptable salts of said compounds, stereoisomers and prodrugs. The present invention also provides intermediates for the preparation of compounds of formula (I), compounds of formula (I), stereoisomers and prodrugs thereof, and pharmaceutically acceptable salts of said compounds, stereoisomers and prodrugs Combination with anti-obesity agents.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Methods and compositions for the "browning" of white fat
InactiveUS20140057837A1Increase volumeIncreases the amount of brown adipose tissueBiocidePeptide/protein ingredientsAgonistBrown adipose tissue
Methods for increasing the amount of brown adipose tissue in a subject, of increasing the ratio of brown fat to white fat in a subject, or for effecting a change in white adipose tissue to become brown adipose tissue in a subject include the administration of one or more agents. The agent can increase or induce hypothalamic expression of BDNF, can be a TrkB receptor agonist or a beta-3 adrenergic receptor agonist, or encode an agonist that modulates a hypothalamic-adipocyte axis.
Owner:DURING MATTHEW +1
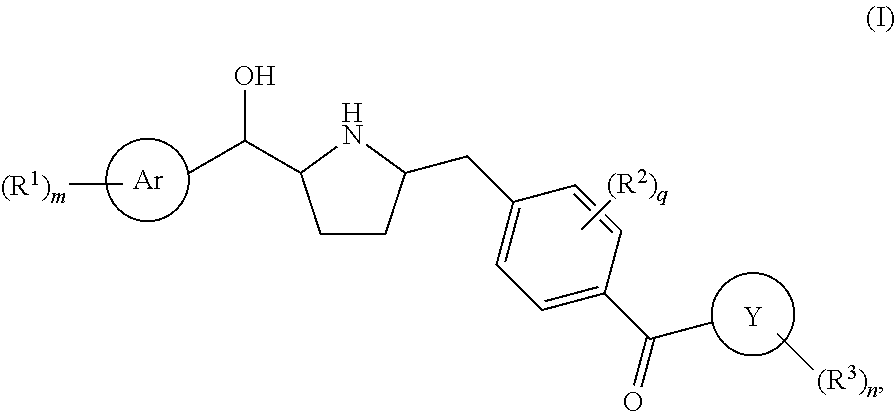
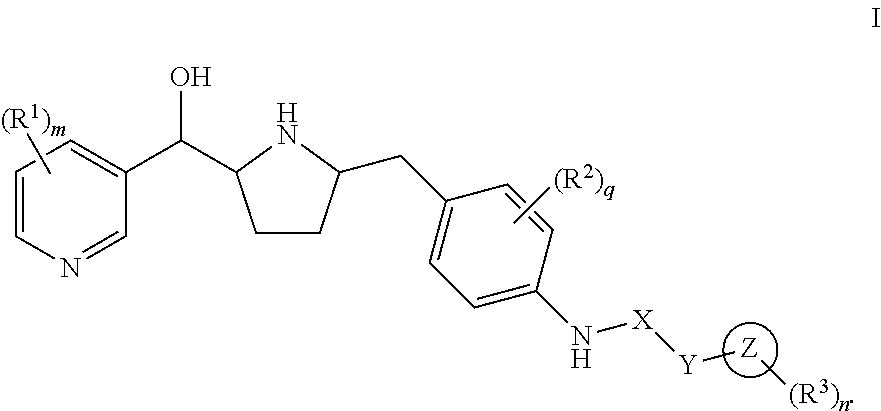
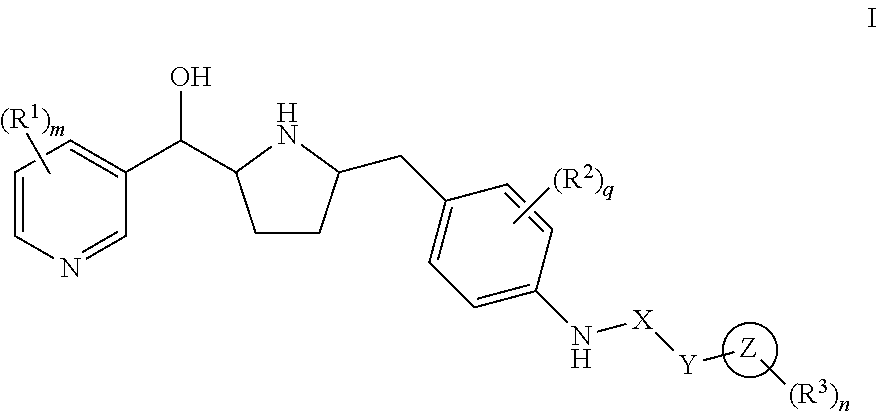
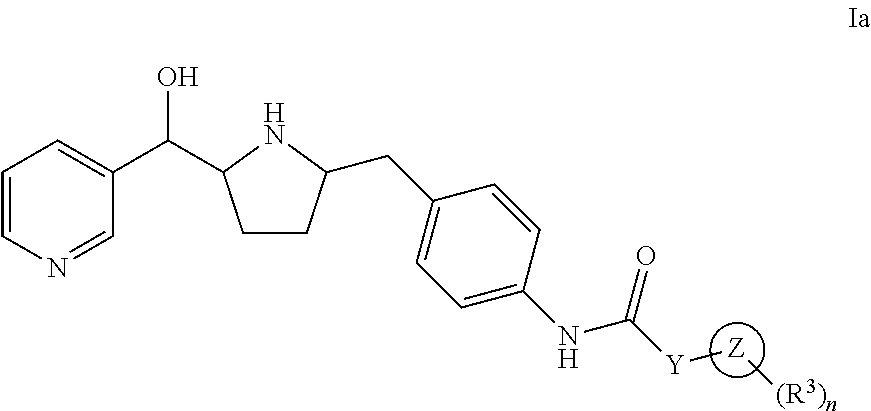
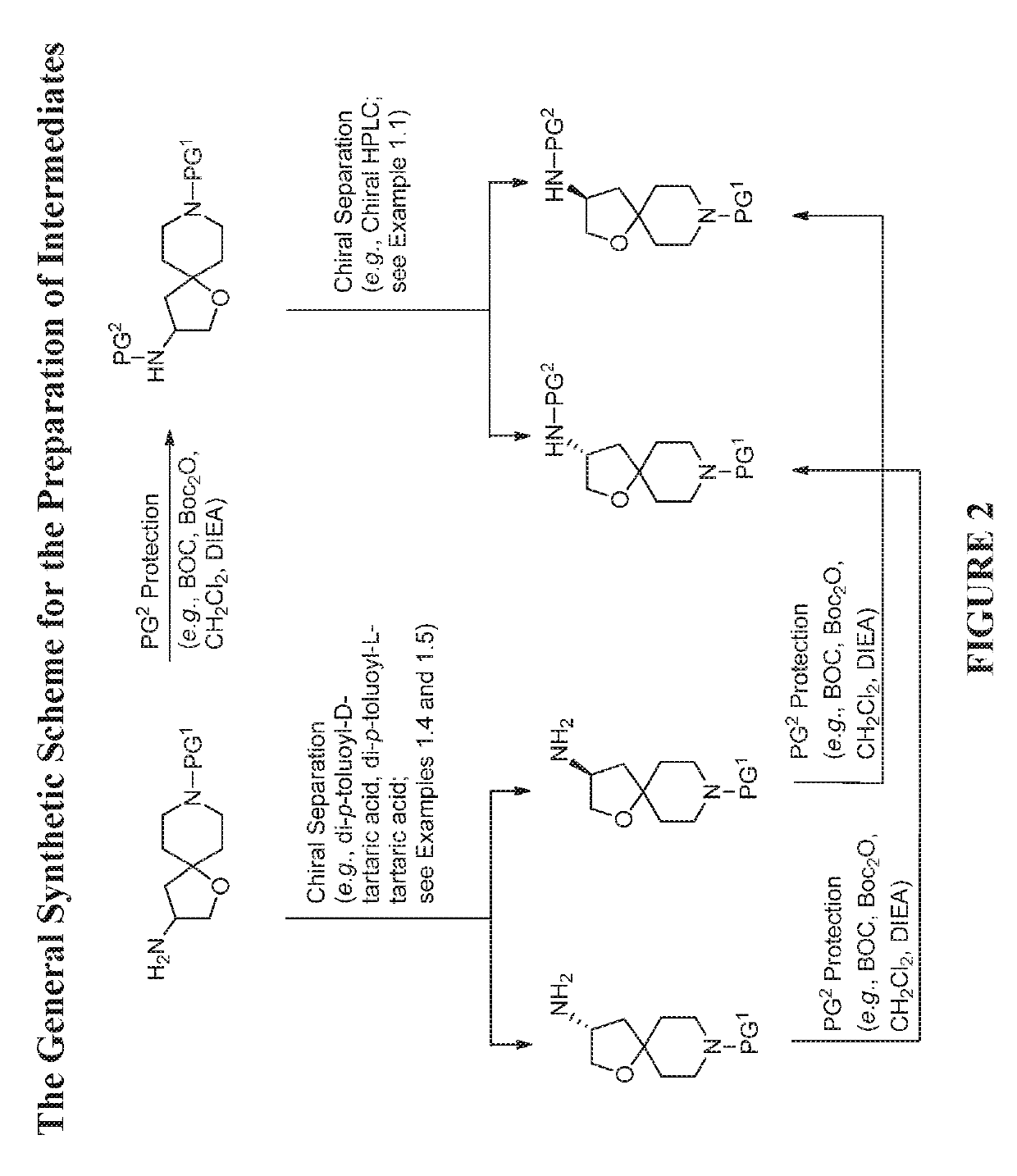
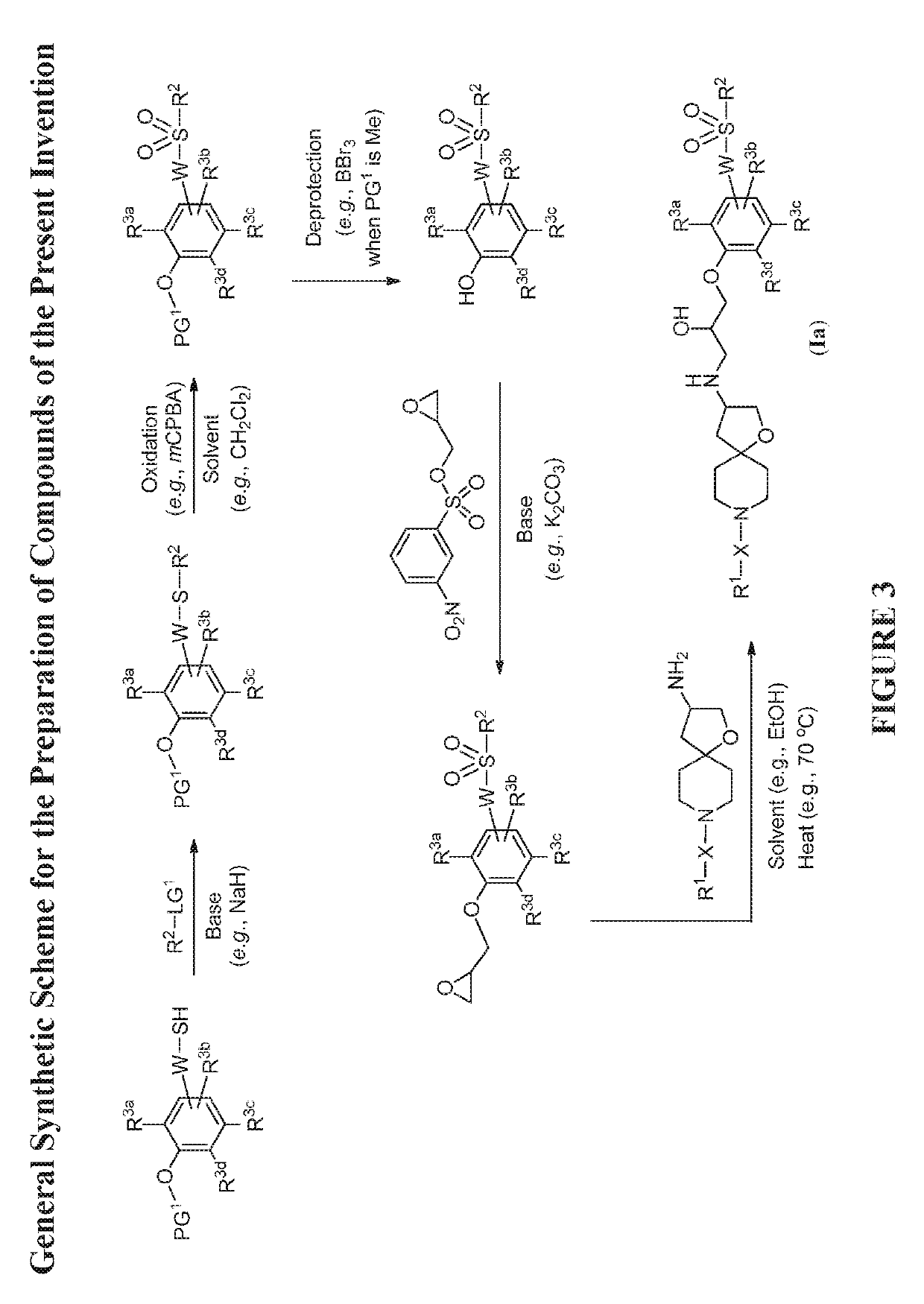
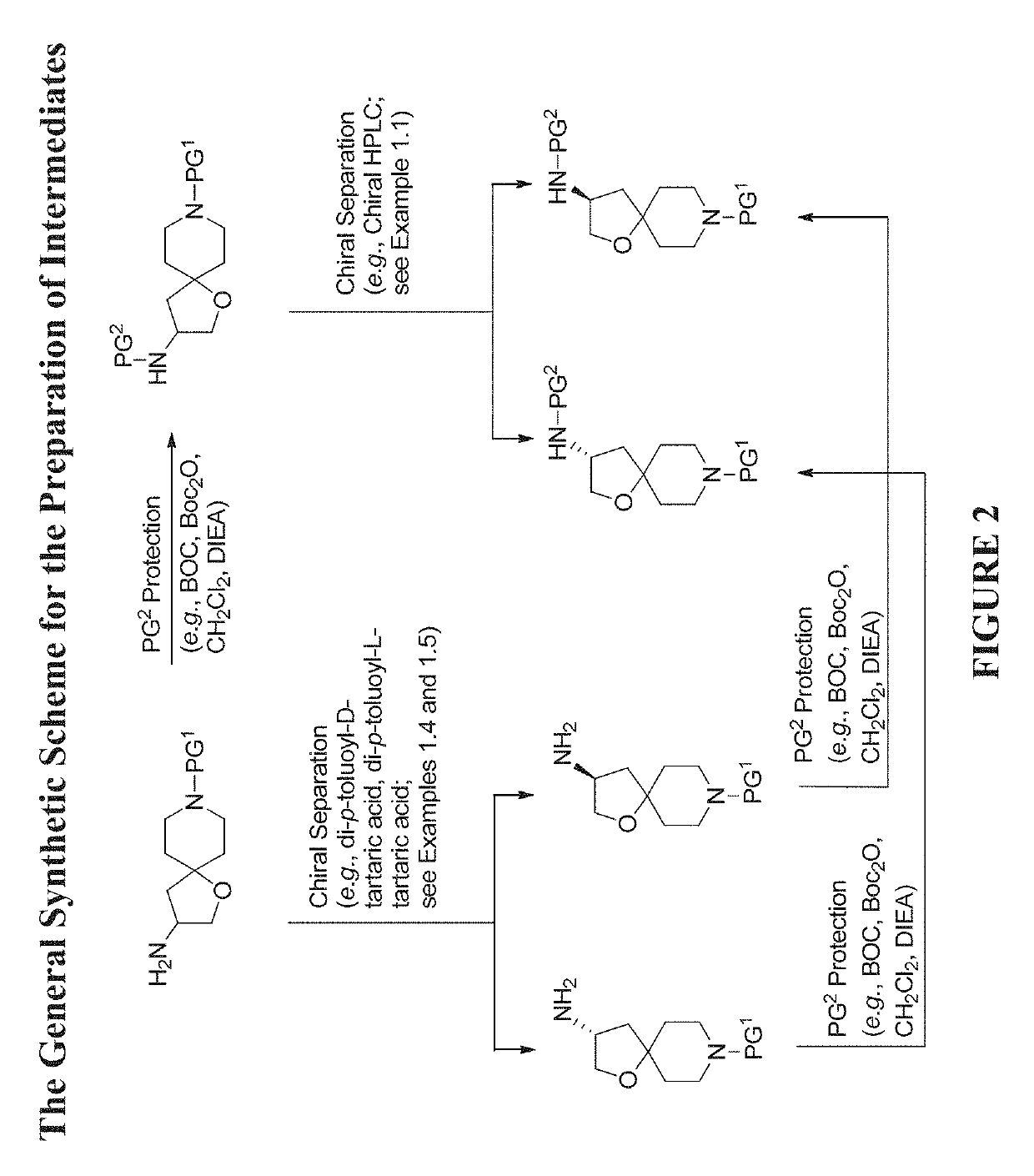
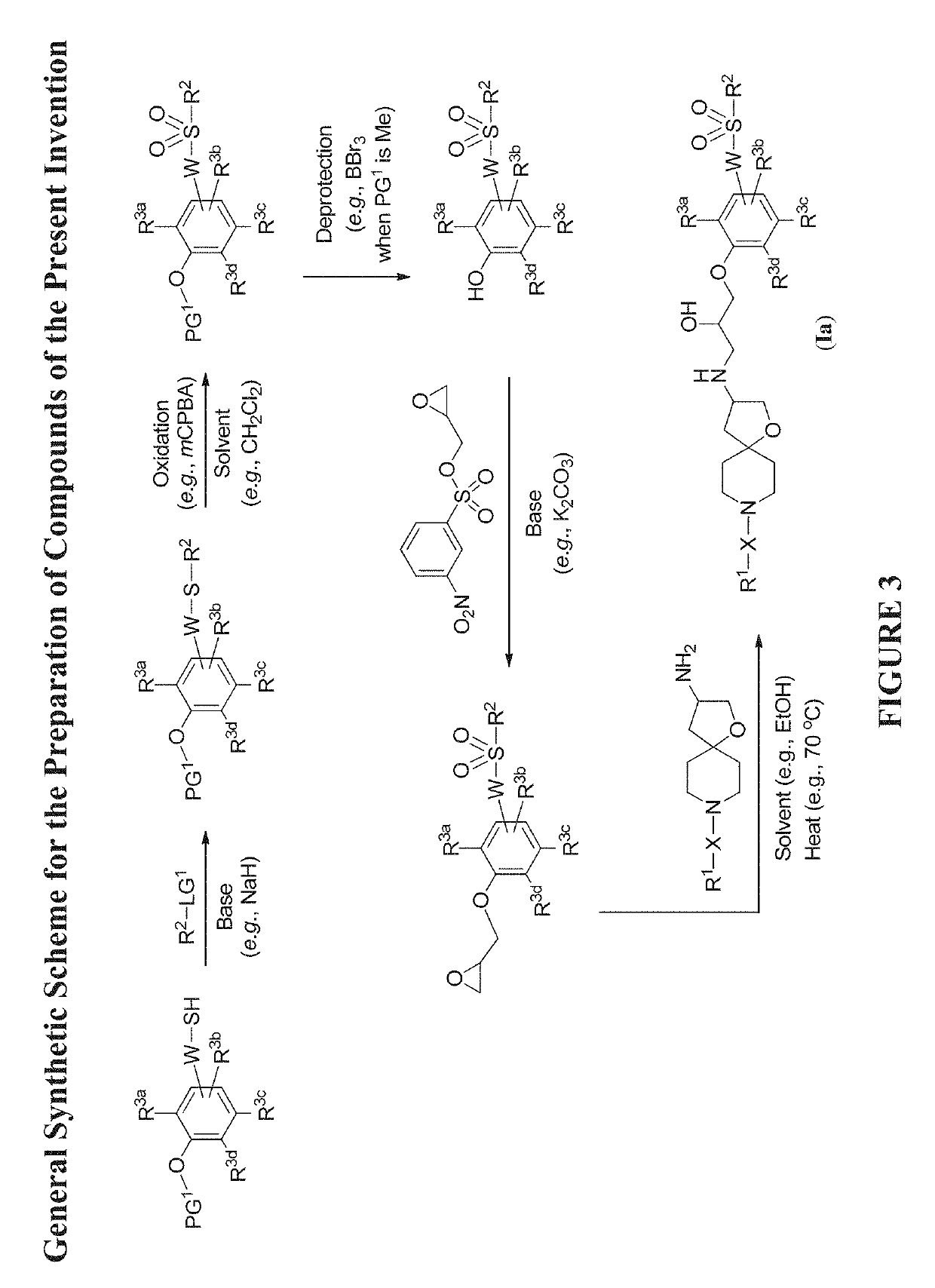
Modulators of the beta-3 adrenergic receptor useful for the treatment or prevention of disorders related thereto
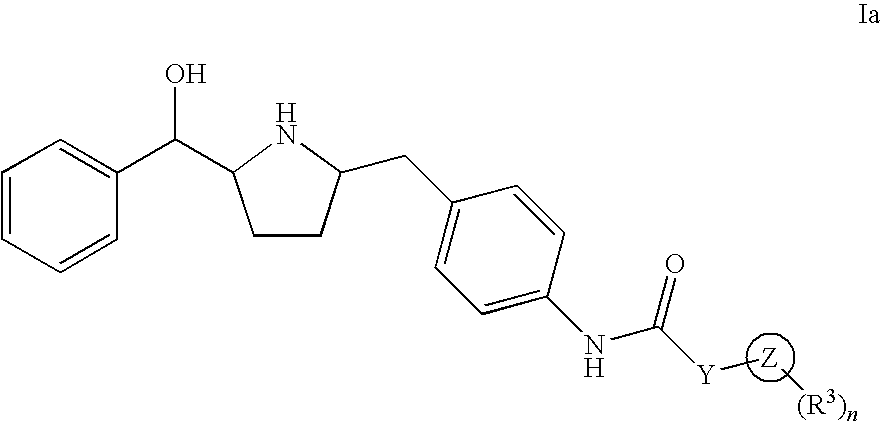
InactiveUS20190284200A1Inhibition of contractilityImproves contractile functionOrganic active ingredientsOrganic chemistryLeft ventricular sizeKidney
The present invention relates to compounds of Formula (Ia) and pharmaceutical compositions thereof that modulate the activity of the beta-3 adrenergic receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of a beta-3 adrenergic receptor-mediated disorder, such as, heart failure; cardiac performance in heart failure; mortality, reinfarction, and / or hospitalization in connection with heart failure; acute heart failure; acute decompensated heart failure; congestive heart failure; severe congestive heart failure; organ damage associated with heart failure (e.g., kidney damage or failure, heart valve problems, heart rhythm problems, and / or liver damage); heart failure due to left ventricular dysfunction; heart failure with normal ejection fraction; cardiovascular mortality following myocardial infarction; cardiovascular mortality in patients with left ventricular failure or left ventricular dysfunction; left ventricular failure; left ventricular dysfunction; class II heart failure using the New York Heart Association (NYHA) classification system; class III heart failure using the New York Heart Association (NYHA) classification system; class IV heart failure using the New York Heart Association (NYHA) classification system; LVEF<40% by radionuclide ventriculography; LVEF≤35% by echocardiography or ventricular contrast angiography; and conditions related thereto.
Owner:ARENA PHARMA
Selective beta -3 adrenergic agonists
The present invention is in the field of medicine, particularly in the treatment of Type II diabetes and obesity. More specifically, the present invention relates to selective beta 3 adrenergic receptor agonists useful in the treatment of Type II diabetes and obesity. The invention provides compounds and method of treating type II diabetes, comprising administering to a mammal in need thereof compounds of the Formulas I and II:
Owner:ELI LILLY & CO
Exercise and diet program
A method of individualized weight management for a subject includes obtaining a biological sample from the subject; detecting the presence or absence of polymorphisms associated with at least seven genes comprising fatty acid-binding protein 2 (FABP2), peroxisome proliferator-activated receptor gamma (PPARG), beta-2-adrenergic receptor (ADRB2), beta-3-adrenergic receptor (ADRB3), angiotensin-converting enzyme (ACE), alpha-actinin-3 (ACTN3), and proton-linked monocarboxylate transporter (MCT1) in the biological sample to obtain genotype pattern data for the subject; wherein the polymorphisms are indicative of at least one nutritional trait and at least one fitness trait and preparing a nutritional and fitness program based on the subject's genotype pattern data; wherein the fitness program comprises sequences of resistance, cardio, and excess post-exercise oxygen consumption (EPOC) training routines.
Owner:ALDO CONSULTING & PROJECT MANAGEMENT
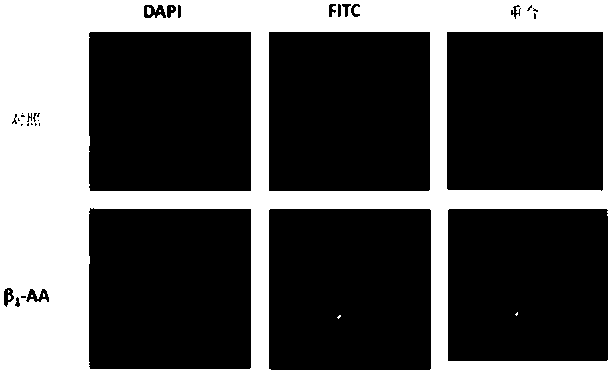
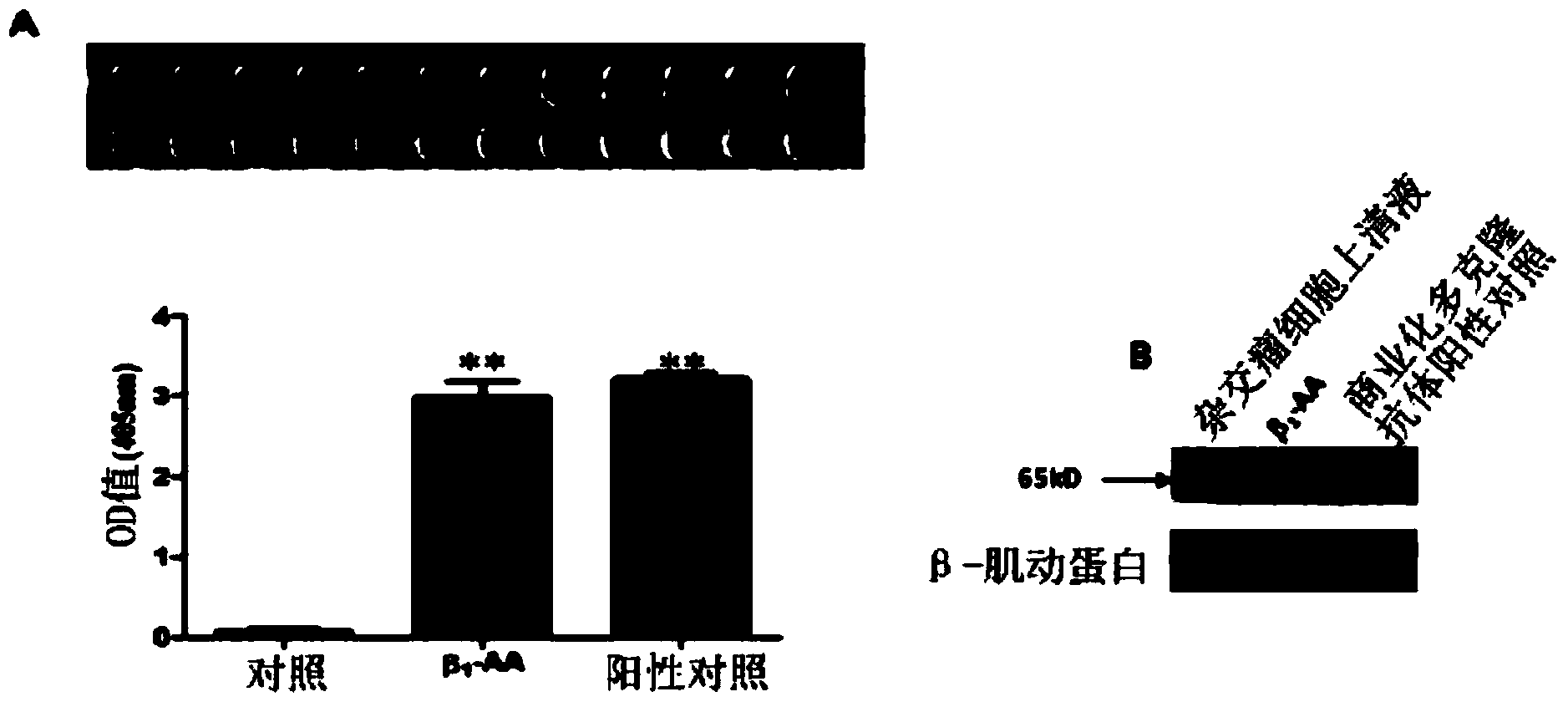
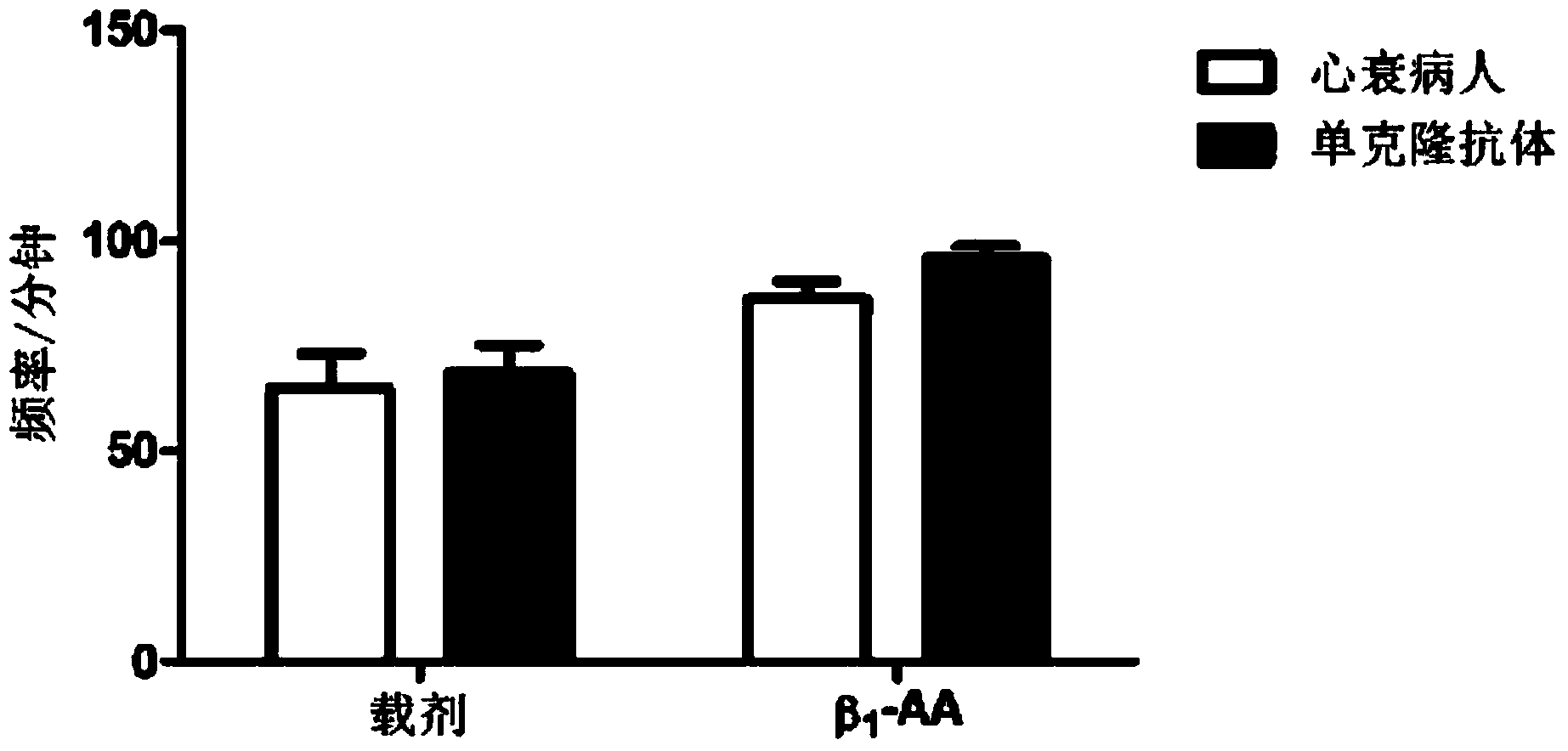
Beta-1 adrenergic receptor monoclonal antibody for inducing myocardial cell apoptosis
InactiveCN103626873ALow immunogenicityImproving immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsSerum igeApoptosis
The invention provides a beta-1-AA monoclonal antibody. The monoclonal antibody is produced by using the following two polypeptides as an immunogen, in which polypeptide 1 is a polypeptide with a sequence having 85%-100% identity with HWWRAESDEARRCYNDPKCCDFVTNRC; polypeptide 2 is a polypeptide with a sequence having 85%-100% identity with CHWWRAES DEARR. The beta-1-AA monoclonal antibody is immunized by using two peptide fragments, wherein the polypeptide 1 fragment is an amino acid sequence on a second ring outside a beta-1 adrenergic receptor cell but has low immunogenicity; while the polypeptide 2 fragment is a peptide fragment with high immunogenicity and is designed for the polypeptide 1 fragment. Therefore, the immunogenicity is improved while guaranteeing acquisition of a correct antibody when the monoclonal antibody is immunized by using the two peptide fragments respectively. The monoclonal antibody which aims at the second ring outside the beta-1 adrenergic receptor cell and has the same biological effect as beta-1-AA in the serum of a clinical patient is successfully prepared for the first time. A reliable experiment result can be obtained when the monoclonal antibody is used in scientific research.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
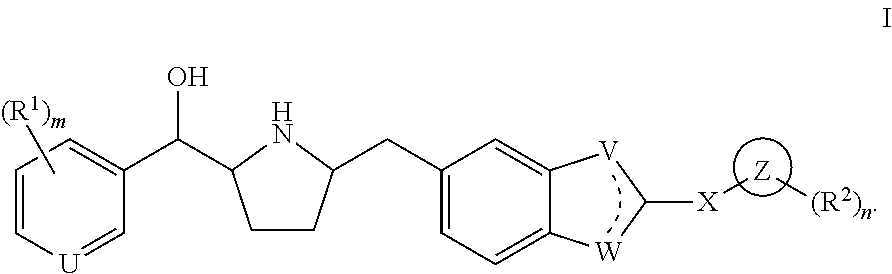
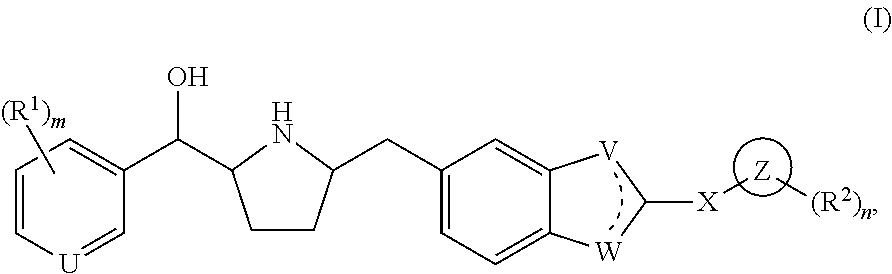
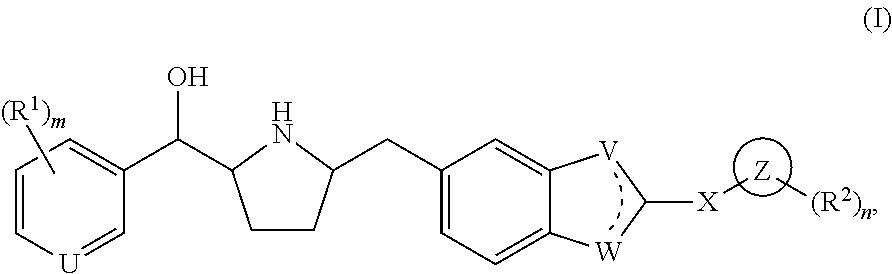
Modulators of the beta-3 adrenergic receptor useful for the treatment or prevention of disorders related thereto
ActiveUS20190292196A1Lower performance requirementsImproves contractile function and hemodynamic statusOrganic active ingredientsOrganic chemistryLeft ventricular sizeKidney
The present invention relates to compounds of Formula (Ia) and pharmaceutical compositions thereof that modulate the activity of the beta-3 adrenergic receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of a beta-3 adrenergic receptor-mediated disorder, such as, heart failure; cardiac performance in heart failure; mortality, reinfarction, and / or hospitalization in connection with heart failure; acute heart failure; acute decompensated heart failure; congestive heart failure; severe congestive heart failure; organ damage associated with heart failure (e.g., kidney damage or failure, heart valve problems, heart rhythm problems, and / or liver damage); heart failure due to left ventricular dysfunction; heart failure with normal ejection fraction; cardiovascular mortality following myocardial infarction; cardiovascular mortality in patients with left ventricular failure or left ventricular dysfunction; left ventricular failure; left ventricular dysfunction; class II heart failure using the New York Heart Association (NYHA) classification system; class III heart failure using the New York Heart Association (NYHA) classification system; class IV heart failure using the New York Heart Association (NYHA) classification system; LVEF<40% by radionuclide ventriculography; LVEF≤35% by echocardiography or ventricular contrast angiography; and conditions related thereto.
Owner:ARENA PHARMA
Beta-3 adrenoceptor agonists for the treatment of pulmonary hypertension
ActiveUS20150374655A1Good effectImprove responseBiocidePeptide/protein ingredientsIntensive care medicineBeta-3 adrenergic receptor
The invention relates to the use of selective agonists of beta-3 adrenergic receptors for the treatment and / or prevention of pulmonary hypertension.
Owner:HOSPITAL CLINIC DE BARCELONA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com