Beta-1 adrenergic receptor monoclonal antibody for inducing myocardial cell apoptosis
A monoclonal antibody and antibody technology, applied in the field of immunology, can solve the problems of high economic cost, not widely used, low concentration of β1-AA, etc., and achieve the effect of improving immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: peptide segment synthesis:
[0027] According to people beta 1 The amino acid sequence of the second extracellular loop of the adrenoreceptor was synthesized into two antigenic peptides, wherein the sequence of polypeptide 1 is HWWRAESDEARRCYNDPKCCDFVTNRC; the sequence of polypeptide 2 is CHWWRAES DEARR.
Embodiment 2
[0028] Embodiment 2: Monoclonal antibody is synthesized by the method of hybridoma cell fusion:
[0029] 1) Coupling polypeptide 1 and polypeptide 2 to carrier proteins KLH and BSA respectively as immunogens;
[0030] 2) Using the two immunogens to immunize three mice respectively to prepare multiple antiserum;
[0031] 3) Biological activity verification with multiple antiserum;
[0032] 4) selecting 1-2 mice with the highest titer, and performing fusion respectively to prepare monoclonal hybridoma cell lines;
[0033] 5) Screening the obtained hybridoma cells by ELISA method, selecting 1 to 5 high-affinity specific monoclonal antibody hybridoma cell lines, and expanding and culturing them;
[0034] 6) One selected clone was inoculated into mice, ascites was collected and Protein G was purified.
Embodiment 3
[0035] Embodiment 3: the biological activity test of the obtained monoclonal antibody
[0036] Detection of β by ELISA, western blotting and laser confocal technology 1 -AA monoclonal antibody and cell surface β 1 -A combination of AR.
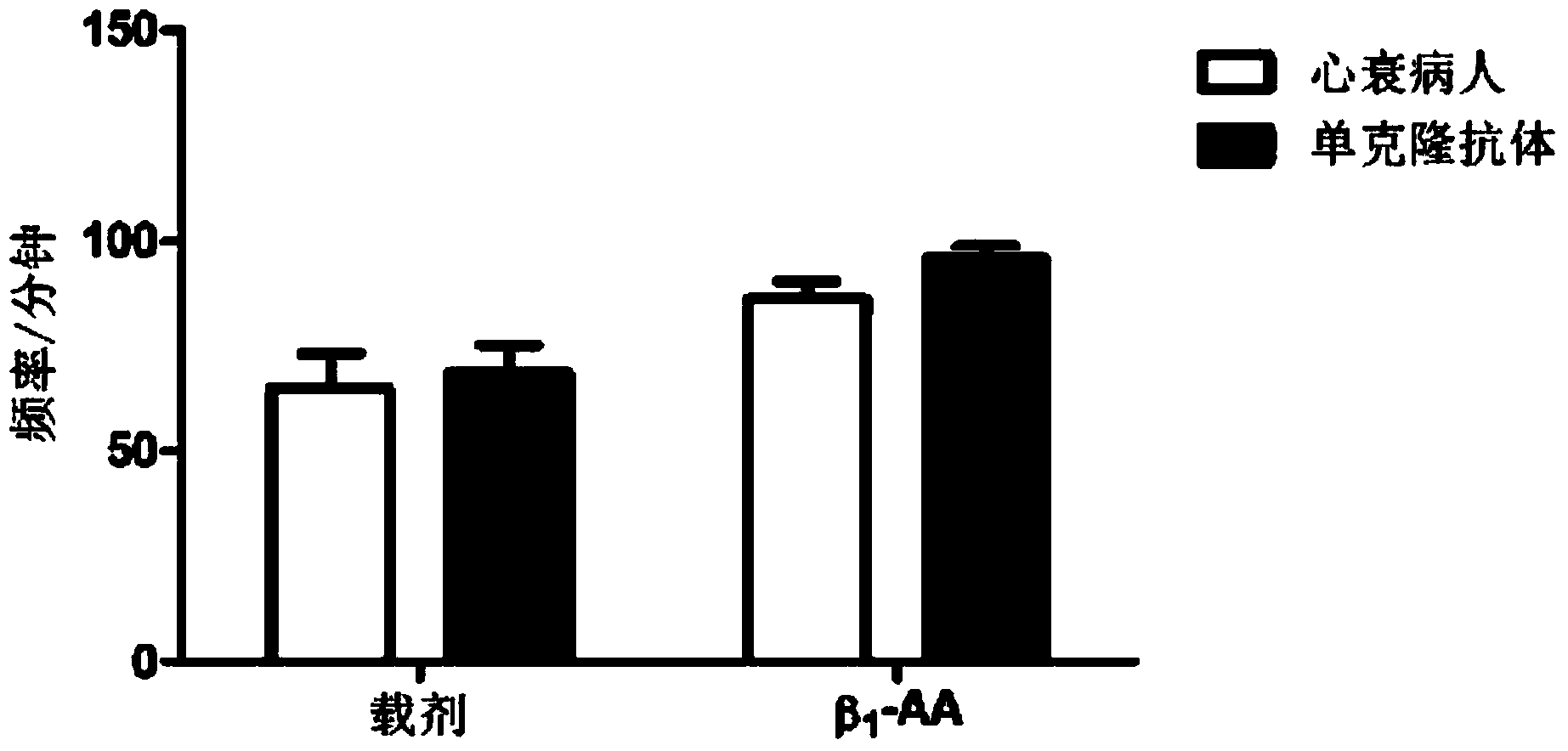
[0037] Experimental evaluation of beating frequency of neonatal rat cardiomyocytesβ 1 - Function of the AA monoclonal antibody.
[0038] Annexin V / PI double staining was detected by flow cytometry β 1 -AA monoclonal antibody against cardiomyocytes H9C 2 Apoptotic effects.
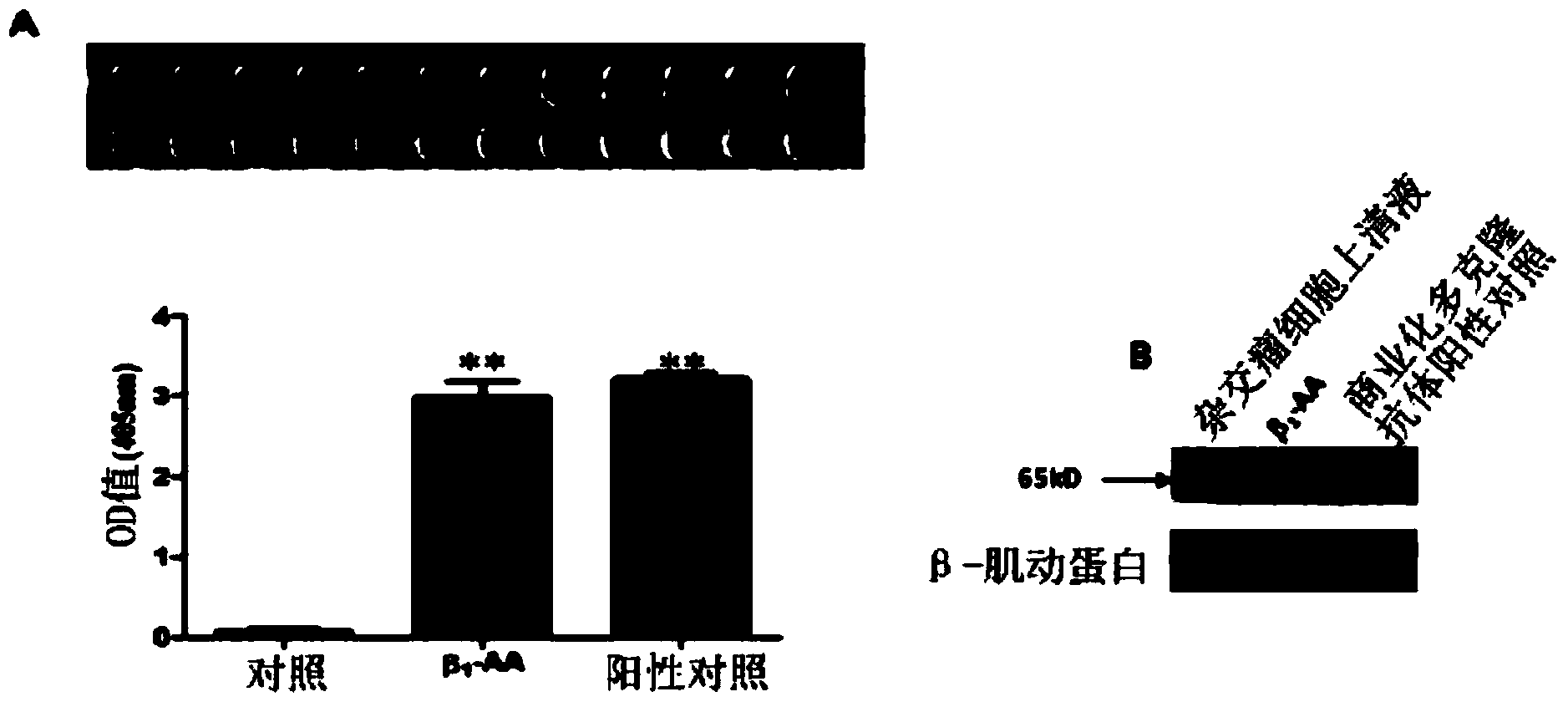
[0039] The results of ELISA detection showed that in the supernatant of hybridoma cells, β 1 The content of -AA was significantly higher than that of the control group (2.9896±0.1873vs.0.0635±0.0386, Pfigure 1 A); and the results of Western blot also confirmed that β 1 -AA monoclonal antibody and commercial anti-β 1 -AR polyclonal antibody positive control has bands with the same molecular weight ( figure 1 B); confocal laser results show that β 1 -AA mAb can inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com