Purine ribosides as antiarrhythmics
A technology for arrhythmia and purine nucleosides, which can be used in drug combinations, pharmaceutical formulations, cardiovascular system diseases, etc., and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
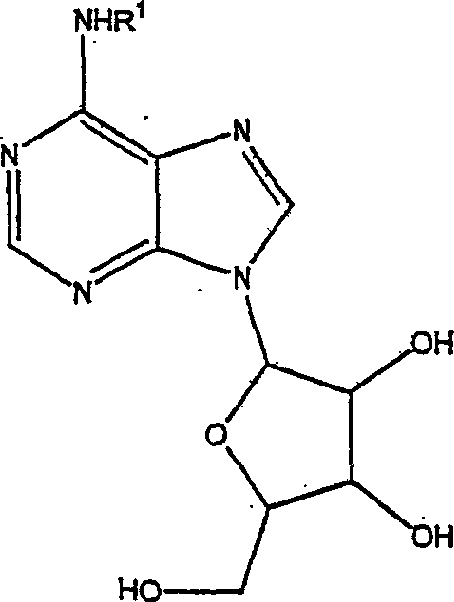
[0052] Example 1 a. Preparation of (3-(S)-aminotetrahydrofuranyl)purine nucleoside step 1. Resolution of 3-(S)-aminotetrahydrofuran
[0053] A mixture of 3-aminotetrahydrofuran hydrochloride (0.5 g, 4 mmol) and (S)-(+)-10-camphorsulfonyl chloride (1.1 g, 4.4 mmol) in pyridine (10 ml) was stirred at room temperature for 4 hours, then concentrate. The residue was dissolved in EtOAc and washed with 0.5N HCl, sodium bicarbonate and brine. The organic layer was dried over magnesium sulfate, filtered and concentrated to give 1.17 g of a brown oil (97%) which was chromatographed on silica gel (25% to 70% EtOAc / Hex). The obtained white solid was repeatedly recrystallized in acetone until 1 HNMR showed that the purity increased to over 90%, and (S)-camphorsulfonate of 3-(S)-aminotetrahydrofuran was obtained. Step 2. Preparation of 3-(S)-aminotetrahydrofuran hydrochloride
[0054] The (S)-camphorsulfonate salt of 3-(S)-aminotetrahydrofuran (170 mg, 0.56 mmol) was dissolved ...
Embodiment 2
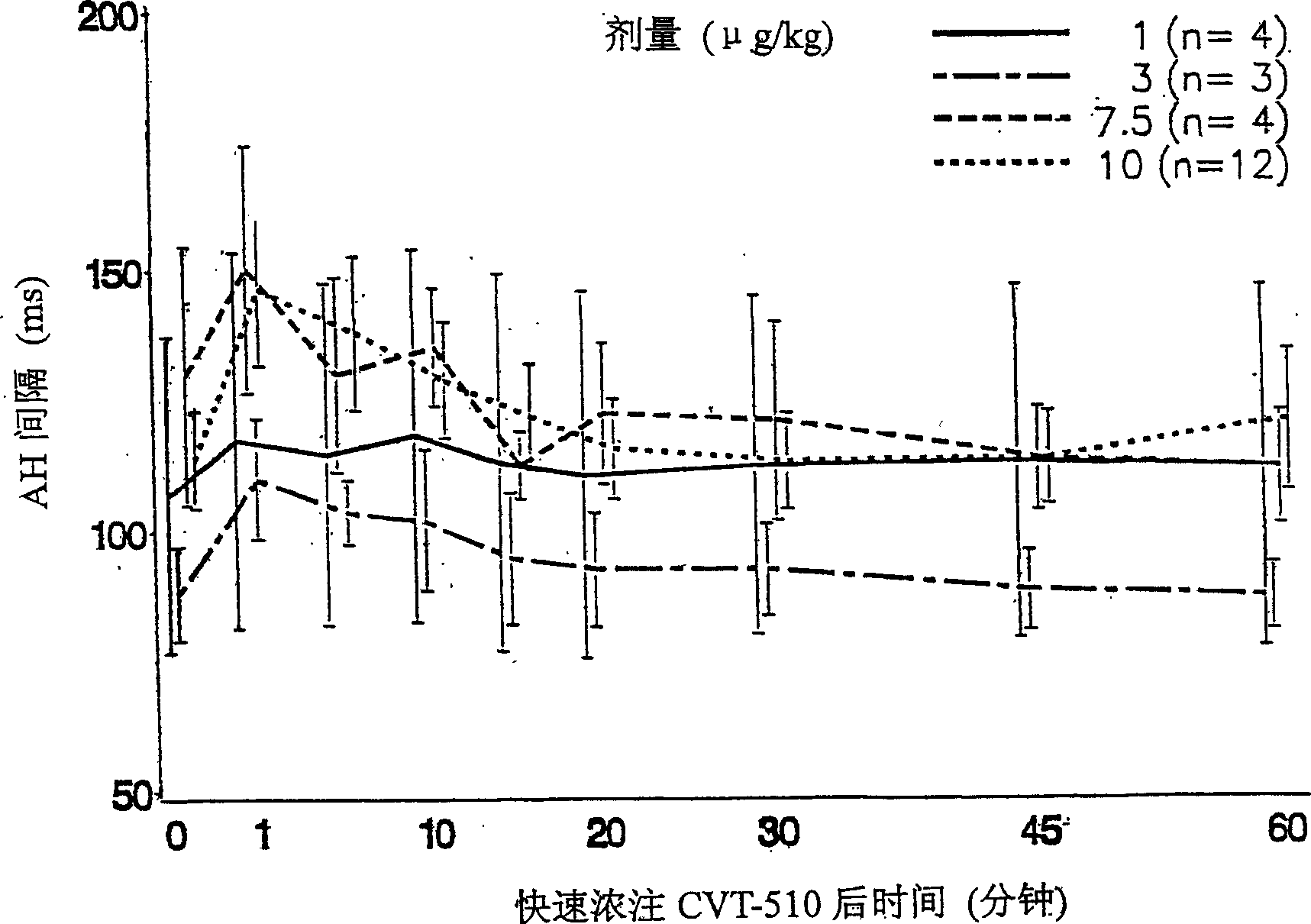
[0059] The effect of CVT-510 on prolongation of the AH interval, which is the adenosine A of CVT-510, and on heart rate (sinus rhythm) in patients with PSVT was tested in patients undergoing clinically indicated electrophysiological studies 1 Measure of the effect mediated. In the first study, a single bolus of CVT-510 was administered to patient volunteers. In the second study, patients were induced with PSVT prior to administration of CVT-510.
[0060] All antiarrhythmic drugs, including digoxin, beta-blockers and calcium channel blockers, were discontinued five half-lives prior to the study.
[0061] The following patients were excluded from the study: if the RR interval was >200 ms or if the resting heart rate was 100 / min. Other exclusion criteria included evidence of ventricular preexcitation, class II-IV congestive heart failure, or asthma. Patients were required to have a normal baseline of electrophysiological parameters, including a Winkbach paced cycle length of 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com