Adenosine A3 receptor agonists
A technology for compounds, chemical formulas, applied in the field of preparing these compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
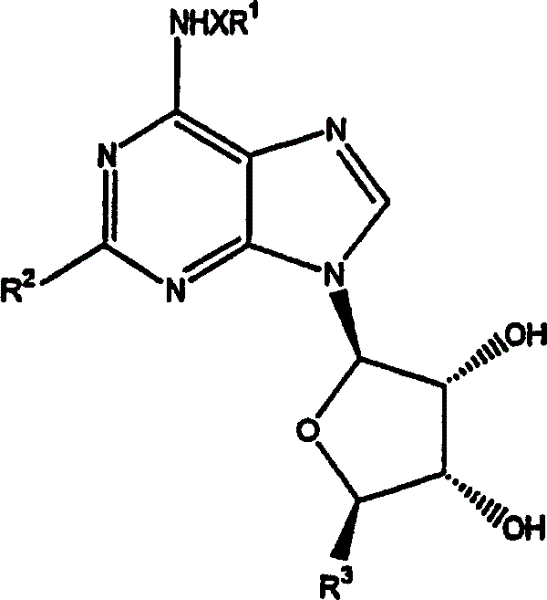
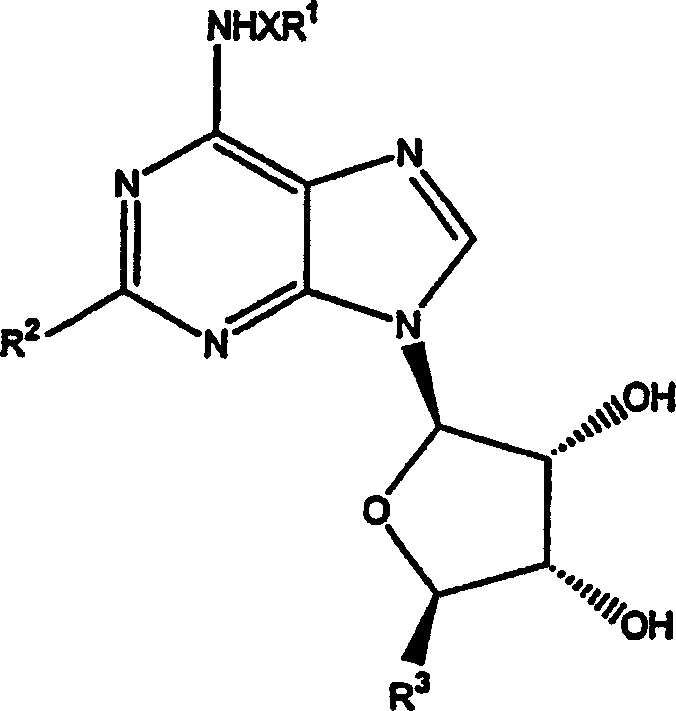
[0176] The preparation of the compound of chemical formula (2)
[0177] a. The preparation of the compound of chemical formula (2), wherein R 1 is a methyl group, and X is a total price bond
[0178]
[0179] 3,4-diacetoxy-2-(2,6-dichloropurin-9-yl)-5-(2-oxopropoxy)tetrahydrofuran, the compound (1mmol) of chemical formula (1) was suspended in 1 :4 in a mixture of methylamine / MeOH and the mixture was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure and the residue was triturated in ether to give (4S,2R,3R,5R)-2-[2-chloro-6-(methylamino)purin-9-yl]-5-(hydroxymethyl ) oxolane-3,4-diol (phenol), a compound of formula (2) in the form of a white solid.
[0180] b. The preparation of the compound of chemical formula (2), change R 1 and x
[0181] Similarly, following the procedure of 1A above, but substituting propylamine and 3-iodoaniline for methylamine, the following compound of formula (2) was prepared: (4S,2R,3R,5R)-2-[...
Embodiment 2
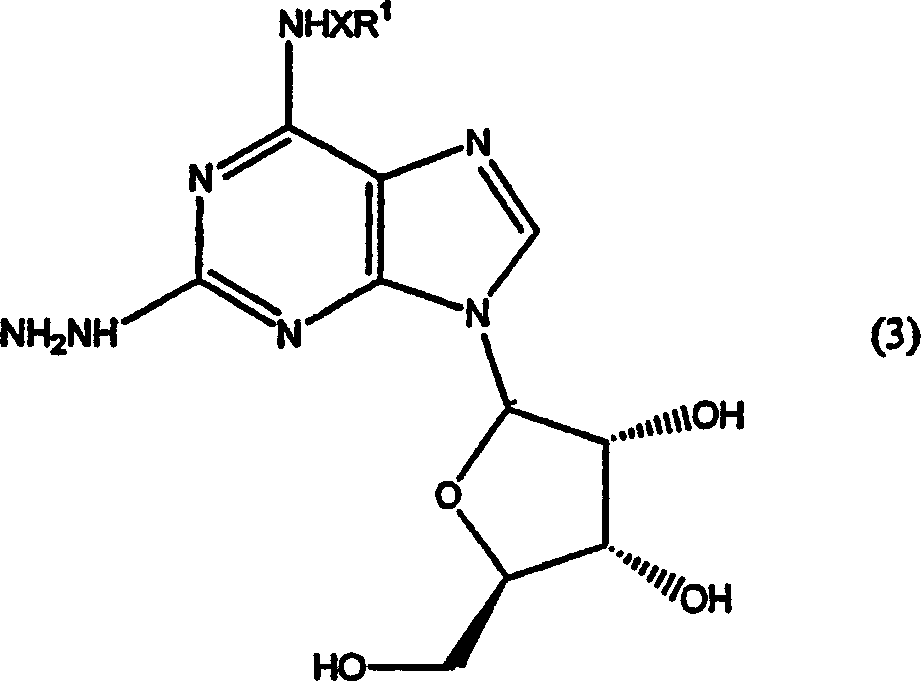
[0198] The preparation of the compound of chemical formula (3)
[0199] a. The preparation of the compound of chemical formula (3), wherein R 1 is a methyl group, and X is a total price bond
[0200]
[0201] (4S, 2R, 3R, 5R)-2-[2-Chloro-6-(methylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol , the compound of chemical formula (2) (0.5 mmol) was suspended in a mixture of hydrazine hydrate (5 mL), and the mixture was stirred at room temperature for 24 hours. Hydrazine was removed under reduced pressure and the residue was triturated in ether and filtered to give (4S,2R,3R,5R)-2-[2-hydrazino-6-(methylamino)purin-9-yl]-5-( Hydroxymethyl)oxolane-3,4-diol, the compound of formula (3) in the form of a white solid.
[0202] b. Preparation of compounds of formula (3), changing R 1 and x
[0203] Similarly, following the procedure of 2A above, but replacing 2-[2-chloro-6-methylaminopurin-9-yl]-5-hydroxymethyltetrahydrofuran-3 with propylamine and 3-iodoaniline ...
Embodiment 3
[0219] Preparation of compounds of formula (I)
[0220] a. The preparation of the compound of chemical formula (I), wherein R 1 is methyl, R 2 for 4-(4- Methoxyphenyl) pyrazol-1-yl, and X is a covalent bond
[0221]
[0222] chemical formula I
[0223] (4S, 2R, 3R, 5R)-2-[2-hydrazine-6-(methylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol (0.5 mmol) was suspended in 3 mL of ethanol, and the compound 2-(4-methoxyphenyl)propionaldehyde of the chemical formula (4) was added to the suspension. The mixture was heated to reflux for 5 hours, and the formed precipitate was collected by filtration, washed with ethanol and ether to give (4S, 2R, 3R, 5R)-5-(hydroxymethyl)-2-{2-[4-( 4-methoxyphenyl) pyrazolyl]-6-(methylamino)purin-9-yl}-oxolane (oxolane)-3,4-diol, the compound of chemical formula I, Ms, 455.43( M+1).
[0224] b. Preparation of Compounds of Formula I, Variation R 1 、R 2 , and X
[0225] Similarly, follow the procedure of 3A above, but option...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com