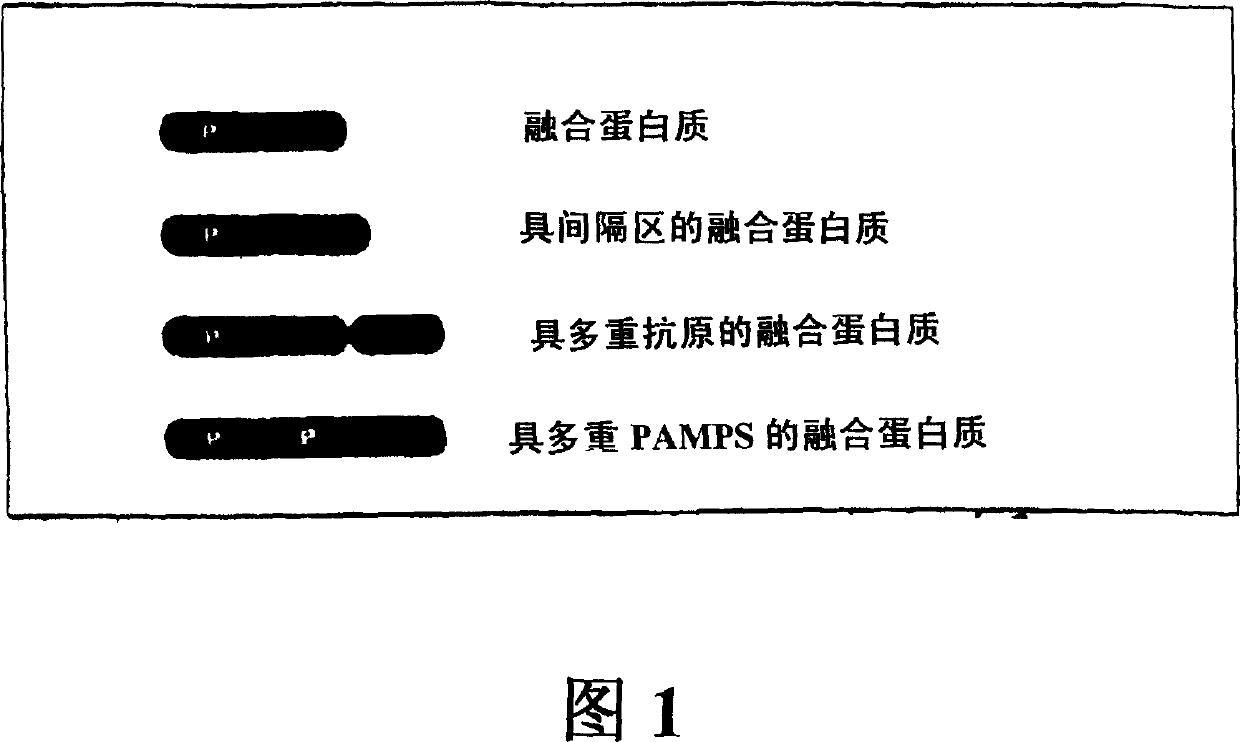
Innate immune system-directed vaccines
A vaccine, immune stimulation technology, applied in allergic diseases, peptides containing affinity tags, resistance to vector-borne diseases, etc., can solve problems such as differences in concepts, strategies and modes of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0240] The preparation of the injectable vaccine of the present invention involves mixing the chimeric construct with muramyl dipeptide or other carrier. The resulting mixture can be emulsified in a mannide monooleate / squalene or squalane vehicle. Four parts squalene and / or squalane are used per part mannide monooleate by volume. Methods of preparing vaccine compositions are well known to those of ordinary skill in the art. (Rola, Immunizing Agents and Diagnostic Skin Antigens. Described in Remington's Pharmaceutical Sciences, 18th Edition, Gennaro (ed.), (Mack Publishing Company 1990) pages 1389-1404).
[0241]Additional pharmaceutical carriers may be employed to control the duration of the vaccine in therapeutic applications. Controlled release formulations can be prepared by using polymers to complex or absorb the chimeric constructs. For example, biocompatible polymers include poly(ethylene-co-vinylacetate) matrices and matrices of polyanhydride copolymers of stearic ac...
Embodiment 1
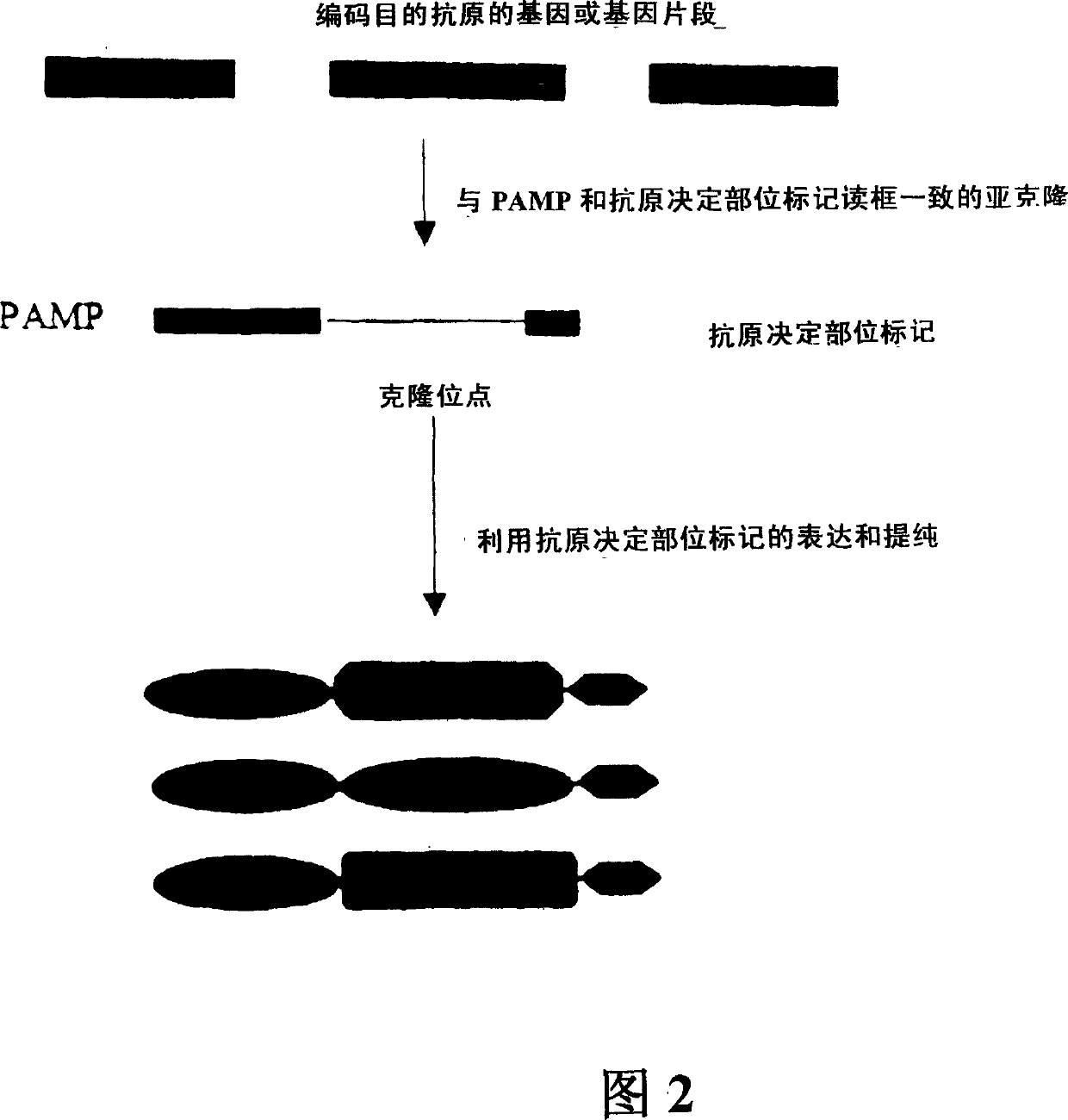
[0266] Example 1. Model vaccine cassette with antigenic domain and PAMP domain
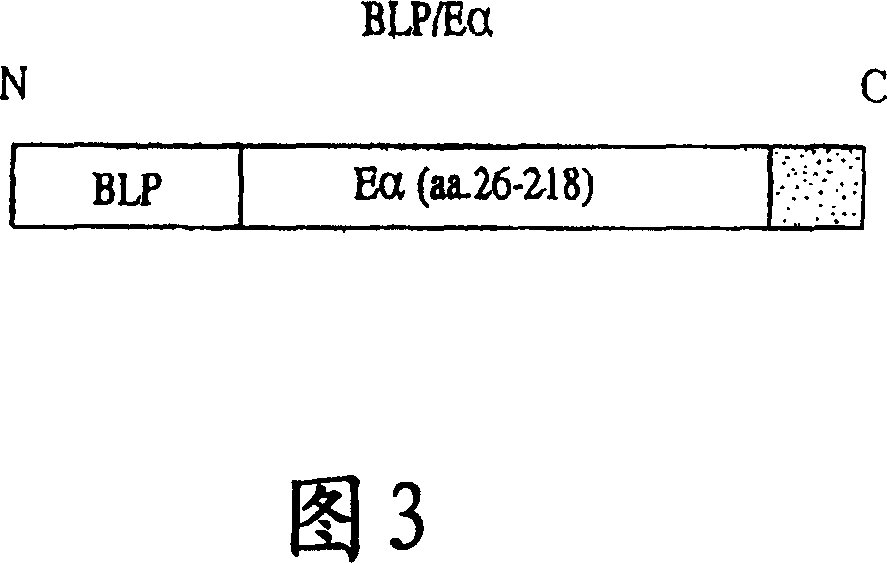
[0267] To produce a model vaccine cassette of the invention, we fused pathogen-associated molecular paradigms (PAMPs) to the characterized mouse antigen Eα. Our chosen PAMP, BLP, is known to stimulate the innate immune system through the receptor Toll-like receptor-2 (TLR-2).
[0268] The bacterial lipoprotein (BLP) protein sequence used in the vaccine cassette for fusion with the antigen of interest is as follows: MKATK LVLGA VILGS TLLAG CSSNA KIDQL SSDVQ TLNAK VDQLS NDVNAMRSDV QAAKD DAARA NQRLD NMATK YRK (SEQ ID NO: 2). The leader sequence includes amino acid 1 to amino acid 20 of SEQ ID NO:2. The first cysteine (amino acid 21 of SEQ ID NO: 2) is lipidated in bacteria. This lipidation, which can only occur in bacteria, is essential for the recognition of BLP by Toll and TLRs. The C-terminal lysine (amino acid 78 of SEQ ID NO: 2) was mutated to increase the yield of the recombinant vaccine...
Embodiment 2
[0271] Example 2. Stimulation of NF-κB by BLP / Eα model antigen in RAW cells
[0272] To test whether model antigens can stimulate signal transduction pathways necessary for immune responses, we measured NF-κB activation in RAW mouse macrophage cell lines in vitro. We developed a stable RAW cell line containing the NF-κB-dependent firefly luciferase gene. Stimulation of these cells by activators of NF-κB results in the production of luciferase, which can be measured in cell lysates using a luminometer. Cells were stimulated with the indicated amounts of BLP / Eα for 5 hours and then harvested for luciferase measurements.
[0273] As a control, RAW cells were stimulated with LPS in the presence and absence of polymyxin B (PmB). PmB can inactivate endotoxin and, as expected, activation of NF-κB activity was reduced by 98% in LPS+PmB samples. BLP / Eα could also activate NF-κB in a dose-dependent manner as shown in Figure 4, however, treatment with PmB did not inactivate the stim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com