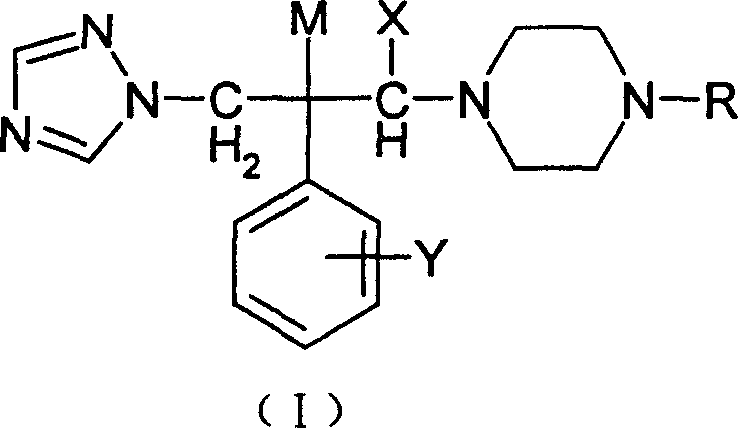
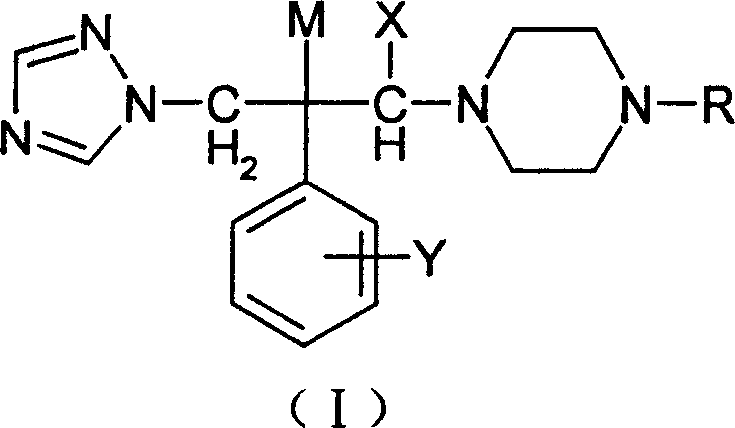
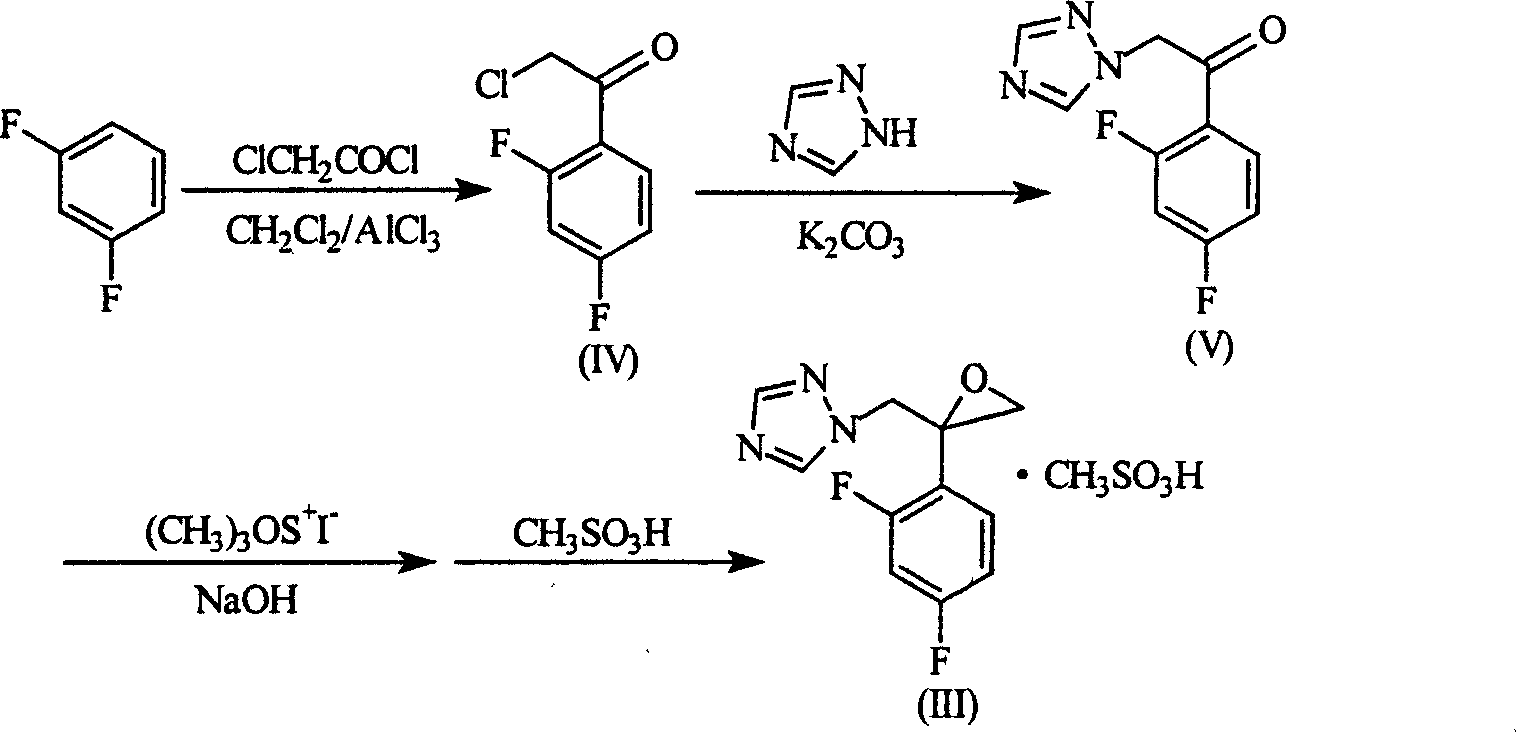
3-substituted piperazine triadimenol antifungal compounds and their salts
A technology of triazole and compound, applied in the field of medicine, can solve the problems of narrow antibacterial spectrum, large toxic and side effects, increase of drug-resistant strains and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[4-(2-furoyl)piperazine]-2- Preparation of Propanol (Compound 1 in Table 1)
[0105] Take 1.65 g (5 mmol) of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole methanesulfonate (III), 1.08g (6mmol) of 2-mercaptopyridine, 2.07g (15mmol) of anhydrous potassium carbonate and 0.1g of phase transfer catalyst hexadecyltrimethylammonium bromide, 30ml of dimethylformamide, in an oil bath at 90-95°C The reaction was heated and stirred for 8 hours. Cool, add 50ml of water under stirring, extract with ethyl acetate (3×70ml), wash with water (30ml×3), anhydrous Na 2 SO 4 Dry, recover the solvent, and recrystallize from absolute ethanol to obtain 1.76 g of a white solid with a melting point of 123-5° C. and a yield of 84.4%.
[0106] 1 H-NMR (DMSO-d 6 )δ, ppm: 8.24 (1H, s, triazole C 3 -H), 7.82 (1H, s, triazoleC 5 -H), 6.77~8.30 (6H, m, Ar-H), 5.10 (1H, s, OH), 4.49-4.58 (2H, AB system, triazole-CH 2 ), 3.33-3.35...
Embodiment 2
[0109] 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[4-(2-(1,2,4-triazole- 1-yl) acetyl) piperazine] -2-propanol (compound 12 in table 1) preparation
[0110] Take 1.65 g (5 mmol) of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole methanesulfonate (III), 2-(1,2,4-triazol-1-yl) acetylpiperazine 1.17g (6mmol), anhydrous potassium carbonate 2.07g (0.015mol) and phase transfer catalyst cetyltrimethylammonium bromide 0.1 g, 25ml of dimethylformamide, heated and stirred in an oil bath at 90-95°C for 8 hours. Cool, add 50ml of water under stirring, extract with ethyl acetate (60ml×3), combine the extracts, wash with water (25ml×3), anhydrous Na 2 SO 4 After drying, filtering, and recovering the solvent, the crude product was obtained, and the crude product was subjected to column chromatography, and the 3 : C 2 h 5 Eluted with OH=9:1, 1.72 g of a light yellow solid was obtained, with a melting point of 121-2°C and a yield of 79.6%.
[0111] 1 H-NMR (DMSO-d ...
Embodiment 3
[0114] 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-[4-(4-(2-pyridinemethoxy)phenyl ) piperazine] the preparation of -2-propanol (compound 16 in table 1)
[0115] Take 1.65 g (5 mmol) of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole methanesulfonate (III), 4-(4-(2-pyridylmethoxy)phenyl)piperazine 1.62g (6mmol), anhydrous potassium carbonate 2.07g (0.15mol) and phase transfer catalyst cetyltrimethylammonium bromide 0.1g , 25ml of dimethylformamide, heated and stirred in an oil bath at 90-95°C for 8 hours. Cool, add 50ml of water under stirring, extract with ethyl acetate (60ml×3), combine the extracts, wash with water (25ml×3), anhydrous Na 2 SO 4 After drying, filtering, and recovering the solvent, the crude product was obtained, and the crude product was subjected to column chromatography, and the 3 : C 2 h 5 Eluted with OH=9:1, 1.32 g of a yellow solid was obtained, with a melting point of 142-4° C. and a yield of 52.2%.
[0116] 1 H-NMR (DMSO-d ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com