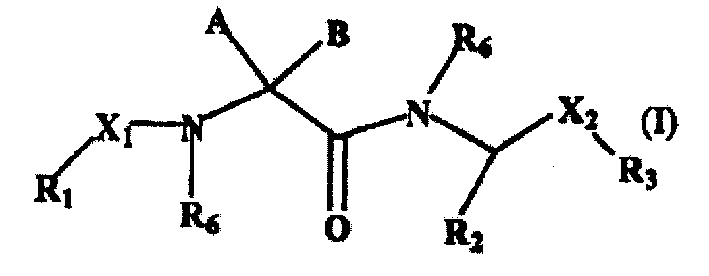
Linear basic compounds having nk-2 antagonist activity and formulations thereof
A compound, alkaline technology, applied in the field of tachykinins, which can solve problems such as excretion and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] N α [N α (Benzo[b]thiophen-2-ylcarbonyl)-1-aminocyclopentane-1-carbonyl]-D-phenylalanine-N-[3-(morpholin-4-yl)propyl] Amide
[0139] 1a) To a solution of 1-amino-cyclopentane-1-carboxylic acid (1 g, 7.66 mmol) in 30 ml of anhydrous dichloromethane (DCM) was added N,O-bis(trimethylsilyl) under magnetic stirring ) acetamide (BSA) (3.8ml, 15.4mmol); after 15 minutes, trimethylchlorosylane (TMSCl) (0.38ml, 10% by volume of BSA) was added. The amino acid was completely sylanised (the solution became clear after the addition was complete), and after stirring for about 2 hours at room temperature, benzo[b]thiophene-2-carbonyl chloride (7.66 mmol) dissolved in 10 ml of DCM was added to the reaction in the mixture. The reaction was continued for 12 hours at room temperature with stirring.
[0140] The solution was concentrated under reduced pressure, then 50 ml of 1M NaHCO was added 3 aqueous solution and stirred for 30 minutes. All was transferred to a separatory funnel,...
Embodiment 2
[0152] (1R, 3S)-N γ {N α [N α (Benzo[b]thiophen-2-yl-carbonyl)-1-aminocyclopentane-1-carboxy]-D-phenylalanyl (alanil)}-3-aminocyclopentane-1-carboxylic acid- N-[(1S,2S)-2-Aminocyclohexyl]amide
[0153] 2a) To a solution of (1S,2S)-diaminocyclohexane (1.14g, 10mmol) in 50ml of DCM was added dibenzyl dicarbonate (2g, 7mmol) dissolved in 20ml of DCM dropwise. After the addition was complete, the resulting precipitate was filtered and dried to afford 0.55 g of a white solid, the starting material diaminecyclohexane. The organic filtrate (80ml) was extracted with 1N HCl (3x10ml) and the aqueous extracts were collected, basified to pH=10 and extracted with DCM (3x10ml). The collected organic extracts were washed with anhydrous Na 2 SO 4 Dry, then filter and dry. The residue was dissolved in 3ml of methanol and then added to ethyl acetate saturated hydrochloric acid (EtOAc / HCl) (2ml) followed by diethyl ether (20ml) to give a suspension with a white precipitate. The precipita...
Embodiment 3
[0172] N γ N γ [N α (Biphenyl-4-ylcarboxy)-1-aminocyclopentane-1-carboxy]-D-phenylalanyl)-(1R,3S)-3-aminocyclopentane-1-carboxylic acid-N- ((1S,2S)-2-aminocyclohexyl)amide trifluoroacetate
[0173] MS-FAB: 664.32(M+H) +
[0174] HPLC (Method E): Rt = 10.5 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com