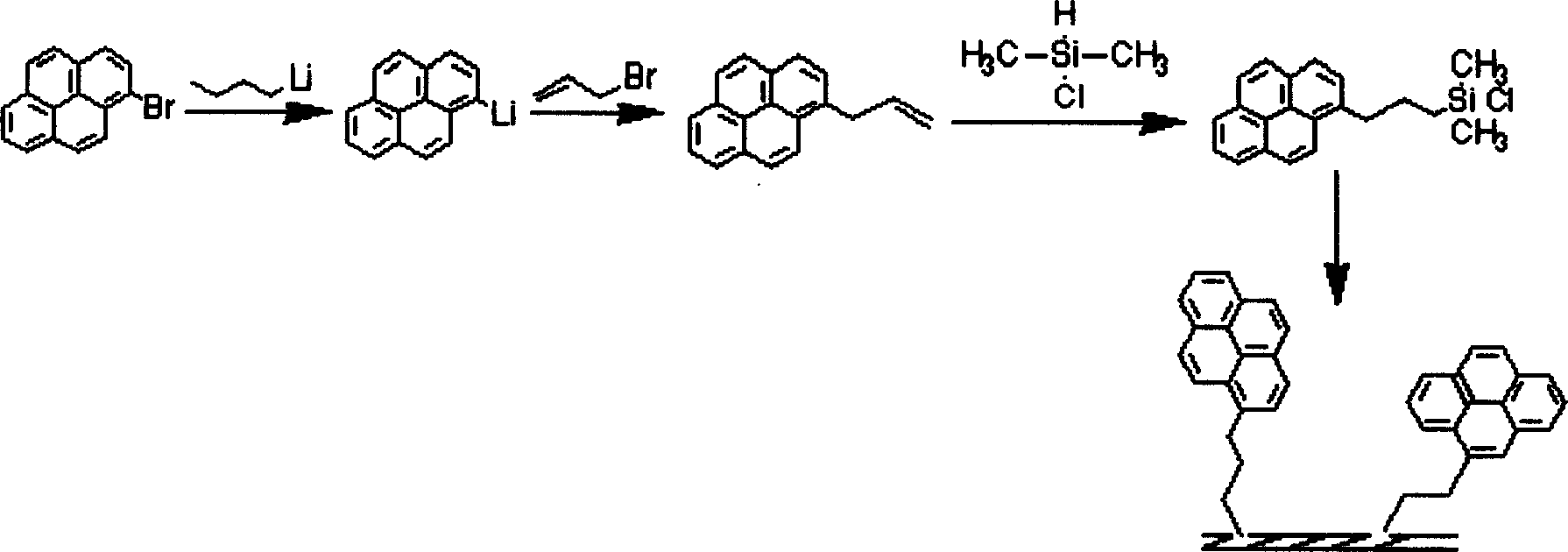
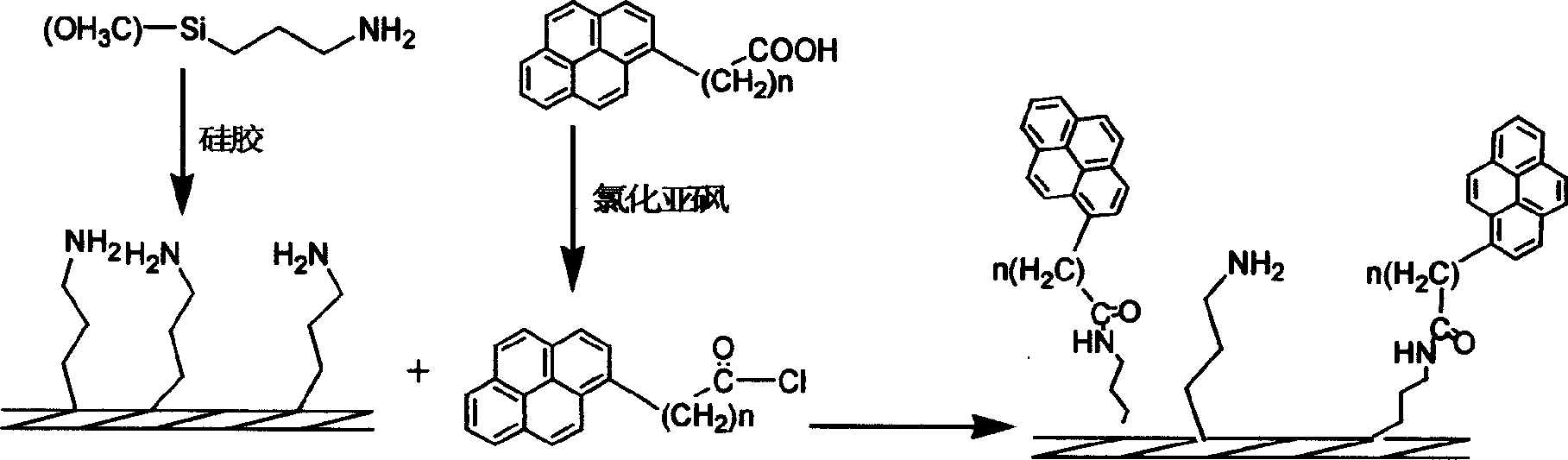
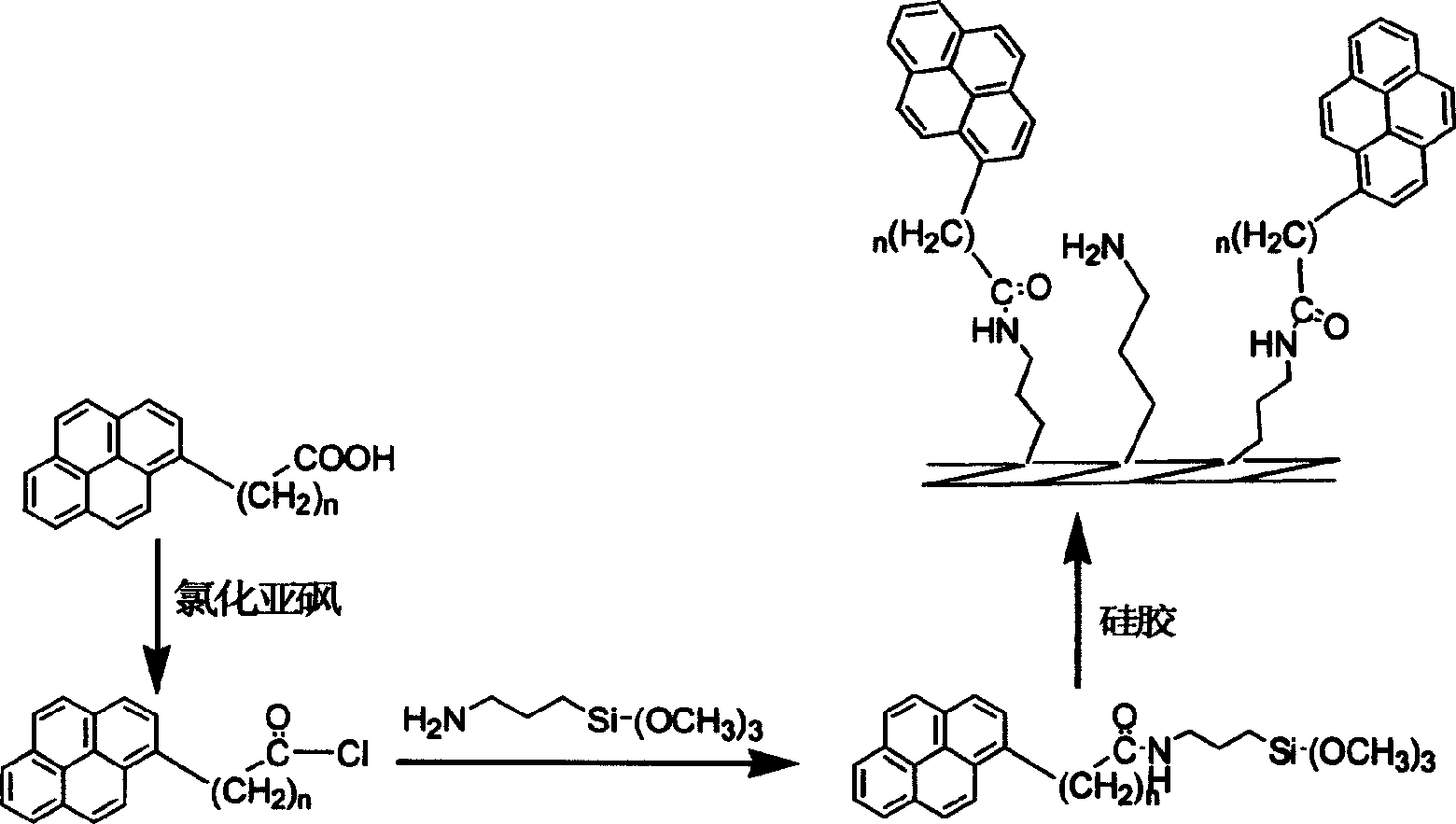
Method for preparing pyrenly bonded silicagel fixed phase
A technology of bonded silica gel and stationary phase, which is applied to instruments, measuring devices, scientific instruments, etc., can solve the problems of complicated and cumbersome preparation and high cost of pyrene-bonded silica gel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Synthesis of pyrenyl butyryl chloride: take 0.5 g of pyrenyl butyric acid solids dried in vacuum in a 50 ml round bottom flask, add 20 ml of dry thionyl chloride, heat and reflux in an oil bath and stir for 24 hours, then distill under reduced pressure. Thionyl chloride is all distilled off, and the remaining solid is pyrenyl butyryl chloride, which is black in color, because it is a macrocyclic aromatic acid chloride, which is not easy to hydrolyze like ordinary small molecular acid chlorides, and is relatively stable in the air. stand-by.
[0021] (2) Amino coupling agent bonded silica gel reaction: Take 2.5g of vacuum-dried silica gel in a 100ml three-necked flask, add 55ml of treated anhydrous toluene and 1.5g of 3-aminopropyltrimethoxysilane, magnetically After stirring, 3 drops (about 0.5 ml) of triethylamine were added dropwise. at N 2 Heated to reflux in an oil bath at 100-120°C in an atmosphere, reacted for 24 hours, cooled, filtered with suction, washed ...
Embodiment 2
[0024] (1) Synthesis of pyrenyl butyryl chloride: take 0.5 g of pyrenyl butyric acid solids dried in vacuum in a 50 ml round bottom flask, add 20 ml of dry thionyl chloride, heat and reflux in an oil bath and stir for 12 hours, then distill under reduced pressure. All the thionyl chloride was distilled off, and the remaining solid was pyrenyl butyryl chloride. stand-by.
[0025] (2) Pyrenylbutyryl chloride and amino coupling agent to generate amide: add 20ml of dry toluene to the pyrenylbutyryl chloride generated in (1), heat to dissolve it completely, pour it into a 100ml three-necked flask, and pass through dry N 2 , cooled, and added dropwise (0.5ml / min) 1.5g of 3-aminopropyltrimethoxysilane and 0.5g of triethylamine in an ice bath, and reacted at room temperature for 12h after 10min.
[0026] (3) Preparation of pyrenyl butyric acid-bonded silica gel: get 2.5g of vacuum-dried silica gel and directly add in the reaction of (2), in N 2 Heat to reflux in an oil bath at 100-1...
Embodiment 3
[0028] (1) Synthesis of pyrenyl acetyl chloride: take 0.5 g of pyrenyl acetic acid solids dried in vacuum in a 50 ml round bottom flask, add 20 ml of dry thionyl chloride, heat and reflux in an oil bath and stir for 12 hours, distill under reduced pressure, and All the thionyl chloride was distilled off, and the remaining solid was pyrenylacetyl chloride for use.
[0029] (2) Amino coupling agent bonded silica gel reaction: Take 2.5g of vacuum-dried silica gel in a 100ml three-necked flask, add 55ml of treated anhydrous toluene and 1.5g of 3-aminopropyltrimethoxysilane, magnetically Stir and add 1 ml of triethylamine. at N 2 Heated to reflux in an oil bath at 100-120°C in an atmosphere, reacted for 24 hours, cooled, filtered with suction, washed with toluene and acetone in turn, and dried in vacuum at 120°C for 5 hours before use.
[0030](3) Preparation of pyrenylacetic acid-bonded silica gel: Take the amino-bonded silica gel in (2) in a 100ml three-necked flask, add it to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com