Method for synthesizing high pure superfine biological glass powder
A bio-glass, high-purity technology, applied in glass molding, glass manufacturing equipment, manufacturing tools, etc., can solve the problems of easy introduction of impurities, environmental pollution, uneven system composition, etc., to achieve hard agglomeration, less introduction of impurities, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
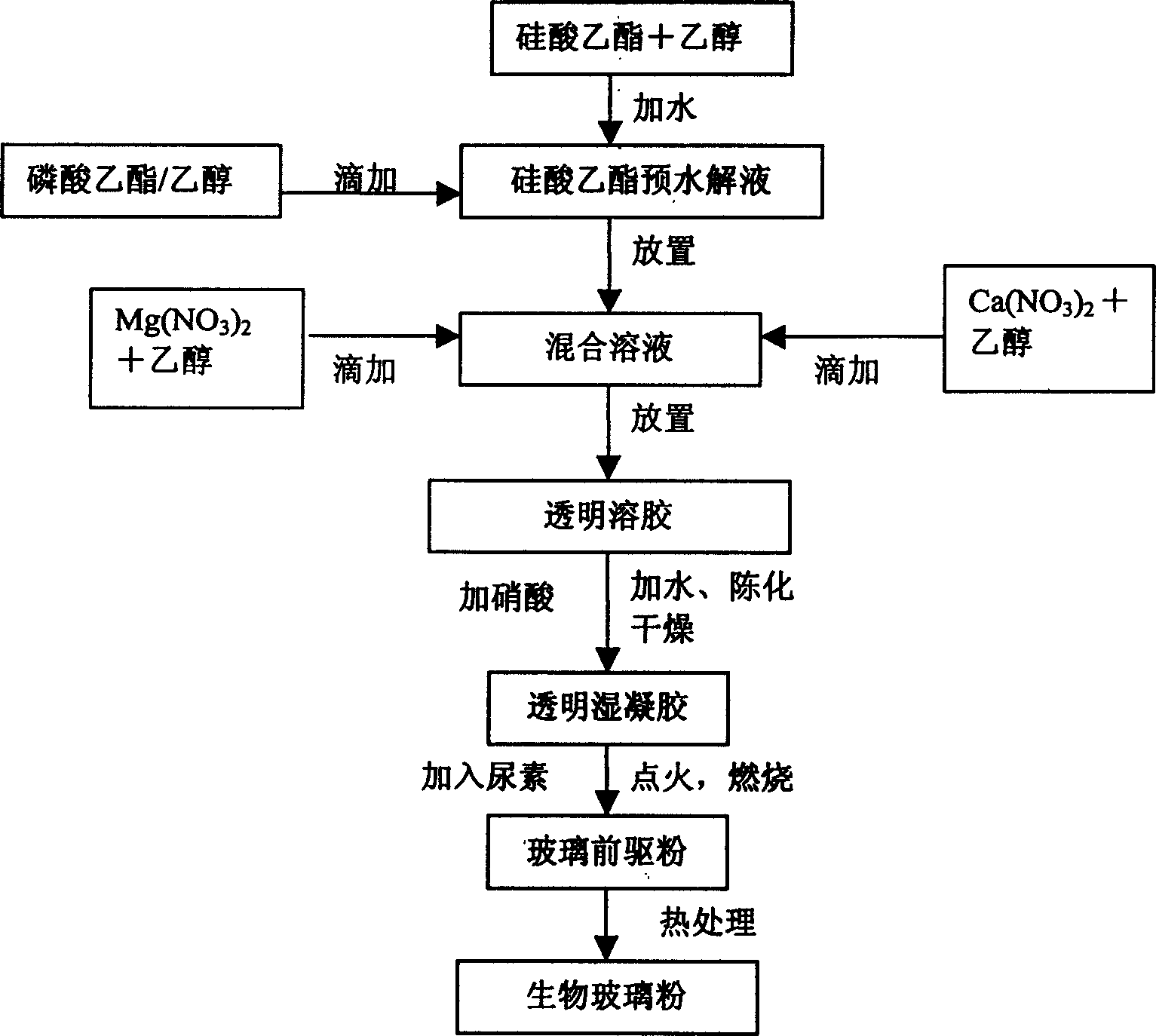
[0020] According to the molar ratio of oxide to SiO 2 :CaO:P 2 O 5 :MgO=5:3:1:1 Measure ethyl silicate, calcium nitrate, ethyl phosphate and magnesium nitrate, and mix them with 10 times the molar ratio of absolute ethanol to obtain ethyl phosphate, Ethanol and ethyl silicate / ethanol, calcium nitrate / ethanol and magnesium nitrate / ethanol solutions. Deionized water with a mole number of 1 times the mole number of ethyl silicate was added dropwise to the ethyl silicate / ethanol solution, and pre-hydrolyzed for 0.5 hours. In the order of ethyl phosphate / ethanol, calcium nitrate / ethanol and magnesium nitrate / ethanol solution, they were added dropwise to the ethyl silicate pre-hydrolyzed solution. After each solution is added dropwise, another solution is added after 1 hour of reaction to ensure the full progress of the reaction. Half an hour after all the solutions were added dropwise, deionized water with a mole number of 1 times the mole number of ethyl silicate was added, and then a...
Embodiment 2
[0022] According to the molar ratio of oxide to SiO 2 :CaO:P 2 O 5 :MgO=1:7:1:1 Measure ethyl silicate, calcium nitrate, ethyl phosphate and magnesium nitrate, and mix them with 9 times the molar ratio of absolute ethanol to obtain ethyl phosphate / Ethanol and ethyl silicate / ethanol, calcium nitrate / ethanol and magnesium nitrate / ethanol solutions. Deionized water with a mole number of 0.5 times the mole number of ethyl silicate was added dropwise to the ethyl silicate / ethanol solution, and it was pre-hydrolyzed for 1 hour. In the order of ethyl phosphate, calcium nitrate / ethanol and magnesium nitrate / ethanol solution, they were added dropwise to the ethyl silicate pre-hydrolyzed solution. After each solution is added dropwise, another solution is added after 0.5 hours of reaction to ensure the full progress of the reaction. Half an hour after all the solutions were added dropwise, deionized water with a mole number of 0.5 times the mole number of ethyl silicate was added, and then...
Embodiment 3
[0024] According to the molar ratio of oxide to SiO 2 :CaO:P 2 O 5 :MgO=7:1:1:1 Measure ethyl silicate, calcium nitrate, ethyl phosphate and magnesium nitrate, and mix them with 8 times the molar ratio of absolute ethanol to obtain ethyl phosphate / Ethanol and ethyl silicate / ethanol, calcium nitrate / ethanol and magnesium nitrate / ethanol solutions. Deionized water with a mole number of 0.5 times the mole number of ethyl silicate was added dropwise to the ethyl silicate / ethanol solution, and it was pre-hydrolyzed for 1 hour. In the order of ethyl phosphate / ethanol, calcium nitrate / ethanol and magnesium nitrate / ethanol solution, they were added dropwise to the ethyl silicate pre-hydrolyzed solution. After each solution is added dropwise, another solution is added after 0.5 hours of reaction to ensure the full progress of the reaction. After adding all the solutions for half an hour, add deionized water whose mole number is 0.5 times the mole number of ethyl silicate, and then add cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com