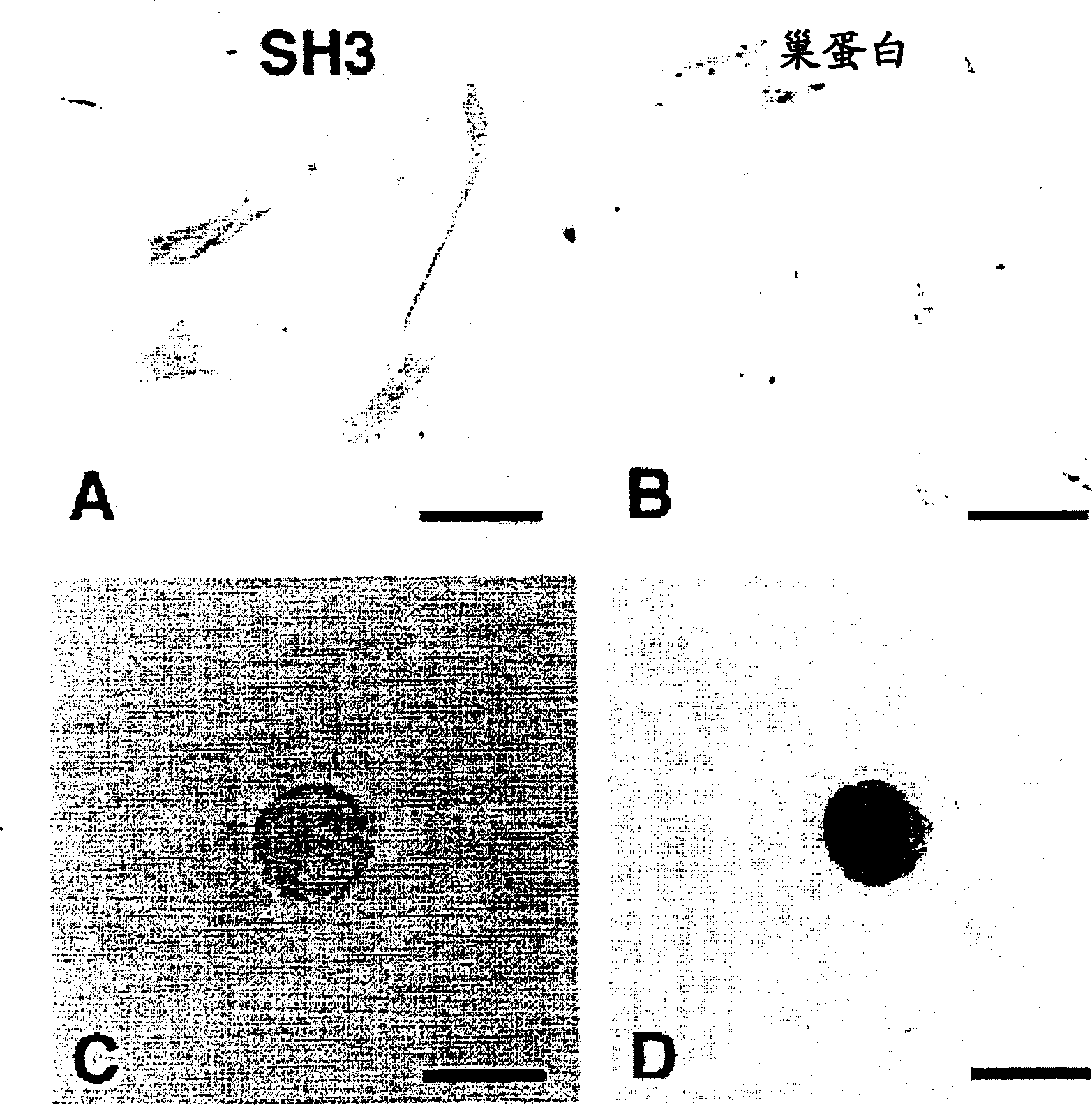
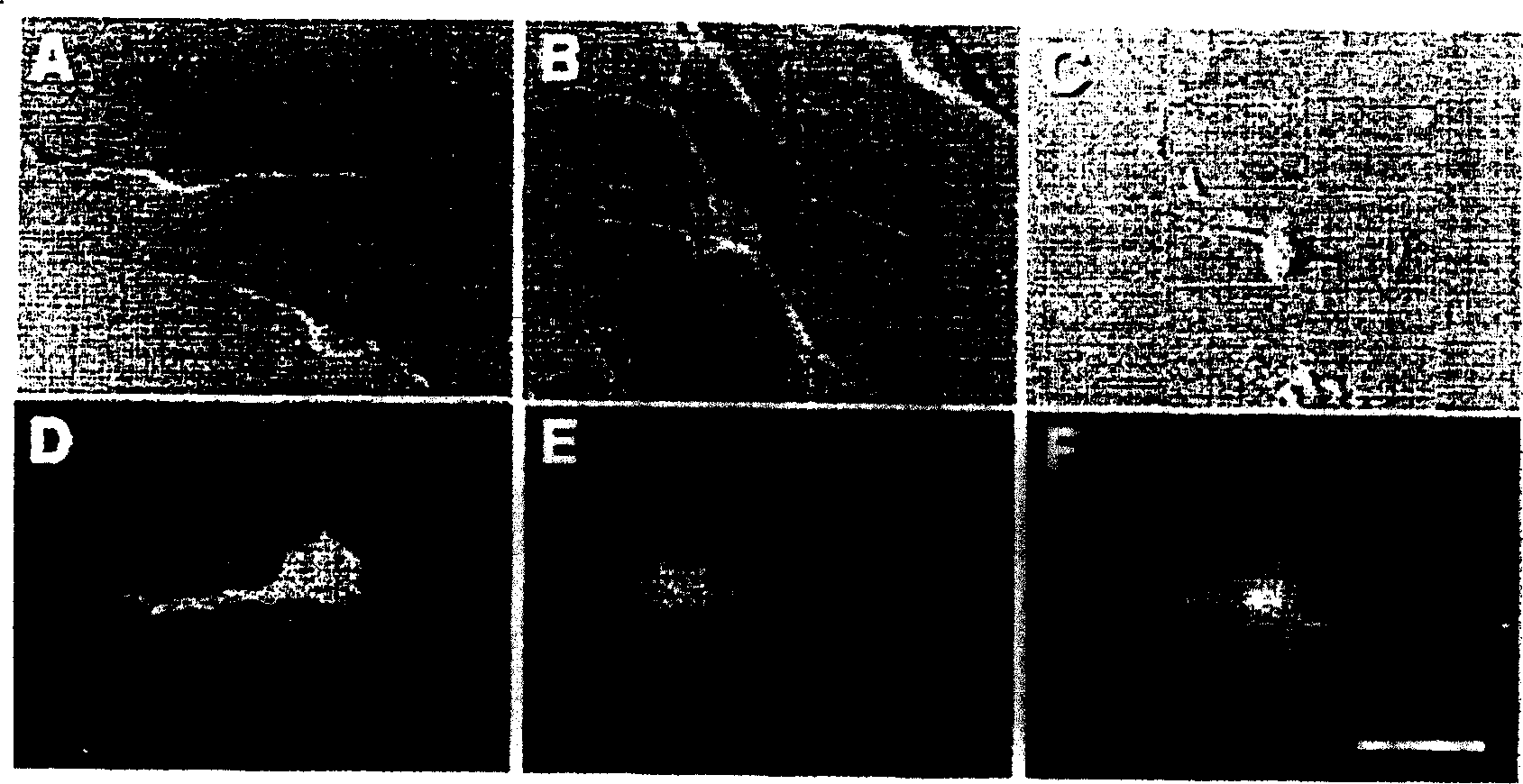
Method of inducing differentiation of mesodermal stem cells, ES cells or immortalized cells into nervous system cells
A stem cell differentiation and nerve cell technology, applied in the direction of non-embryonic pluripotent stem cells, artificially induced pluripotent cells, embryonic cells, etc., can solve the problem of neural stem cell organization difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1: Preparation of monocyte fraction and mesoderm stem cells
[0120] (1) Mononuclear cell part and culture solution of monocyte part:
[0121] Specimens collected from mice (or humans) were diluted with L-15 medium (2 ml) containing 3 ml of Ficoll, and centrifuged at 2,000 rpm for 15 minutes. Cells were isolated from the monocyte fraction and suspended in 2 ml of serum-free medium (Neural Progenitor Maintenance Medium (NPMM)). The suspension was centrifuged at 2,000 rpm for 15 minutes. Discard the supernatant and collect the pelleted cells. The monocyte fraction was prepared by resuspending cells in NPMM.
[0122] Further, the preparation of the culture solution of the monocyte fraction was carried out at 37 °C, 5% CO 2 Incubate the mononuclear cell fraction in the following media under the following conditions: Medium 1 [DMEM (Dulbecco's Modified Eagles Medium-Low Sugar) / 10% FBS (Fetal Bovine Serum) / 1% Antibiotic Antimycotic Solution], Medium 2, and medi...
Embodiment 2
[0125] Example 2: Induction of differentiation of mesoderm stem cells into neural cells
[0126] (1) Induction of differentiation of mesoderm stem cells into neural stem cells:
[0127] First, the cells were washed and then the cells in the medium were treated with enzymes (reagents: 0.05% trypsin / 0.02% EDTA; room temperature for 5 minutes) to collect. After adding an equal volume of medium, pipette several times to disperse the cells into single cells. Cells were then centrifuged at 600 rpm for 5 minutes. Aspirate the pelleted cells with a pipette. Then, the cells were further cultured in suspension in culture vessels (untreated polystyrene plates) at 37°C, 5% CO 2 Under the condition, use fresh following culture medium: Medium 1 [50%DMEM (Dulbecco's modified basal medium) / 50%F-12 / 1%FSC; Daily add 10ng / ml basic fibroblast growth factor ( bFGF) and 10ng / ml epidermal growth factor (EGF)] or in medium 2 [NPBM (neural progenitor cell basal medium; Clonetics) / 2% neural survi...
Embodiment 3
[0132] Example 3: The use of ischemic brain extracts to promote the induction of mesoderm stem cells to differentiate into neural cells
[0133] During the culture of mesodermal stem cells, it was found that the addition of ischemic brain extract, prepared from brain exposed to ischemic stress, allowed mesodermal stem cells to differentiate into neural stem cells more efficiently. This method was found to ensure efficient differentiation of mesoderm stem cells into neural stem cells with only a few days of culture.
[0134] (1) Preparation of ischemic brain extract:
[0135] First, rats were deeply anesthetized with sodium pentobarbital and the precordial region was incised from the anterior abdominal wall to expose the heart and ascending aorta. Cut the apex and cannulate the ascending aorta from the left ventricle. Then, an incision was made in the right atrial appendage of the heart, and the cannula was perfused with saline for 3 minutes to allow sufficient blood outflo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com