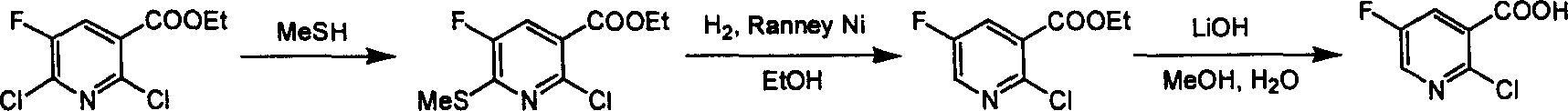Preparation of 2-Cl-5-F-nicotinate and nicotonic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The first step: the synthesis of 2-chloro-5-fluoro-nicotinic acid ethyl ester
[0019] Add triethylamine (32 g, 0.32 mol) and Lindlar catalyst (2.5 g) to ethyl acetate solution (1.2 L) of 2,6-dichloro-5-fluoro-nicotinic acid ethyl ester (50 g, 0.21 mol), Hydrogenation was carried out at 3 atmospheres at room temperature for 12 hours. The catalyst was removed by filtration, the reaction solution was concentrated and then column chromatographed to obtain 2-chloro-5-fluoro-nicotinic acid ethyl ester (24.1 g, 0.12 mol, yield: 55%) as a colorless oil. 1 H NMR (400MHz, CDCl 3 ): δ8.36(d, J=2.8Hz, 1H), 7.88(dd, J=8.0 & 2.8Hz, 1H), 4.40(q, J=7.2Hz, 2H), 1.39(t, J=7.2Hz ,2H); 13 C NMR (400MHz, CDCl 3 ): δ164.5(C), 160.5(C), 158.0(C), 146.0(d, J=10.8Hz, C), 141.3(d, J=99.6Hz, CH), 146.0(d, J=10.8 Hz, C), 129.2(d, J=13.6Hz, C), 128.5(d, J=85.4Hz, CH), 63.9(d, J=48.0Hz, CH 2 ), 15.4 (CH 3 ); Ms(M + +1204206).
[0020] The second step: the synthesis of 2-chloro-5-fluoro...
Embodiment 2
[0023] Synthesis of 2-amino-5-fluoro-nicotinic acid ethyl ester
[0024] Add triethylamine (32 g, 0.32 mol) and 5% Pd-C catalyst ( 1.0 g), hydrogenated at normal pressure and room temperature for 12 hours. The catalyst was removed by filtration, the reaction liquid was concentrated and then column chromatographed to obtain oily 2-chloro-5-fluoro-nicotinic acid ethyl ester (18.4 g, 0.09 mol, yield: 43%).
Embodiment 3
[0026] Synthesis of 2-chloro-5-fluoro-nicotinic acid ethyl ester
[0027] Add triethylamine (32g, 0.32mol) and 5% Raney nickel (1.0 g) Hydrogenation at normal pressure at 40°C for 12 hours. The catalyst was removed by filtration, the reaction solution was concentrated and then column chromatographed to obtain oily ethyl 2-chloro-5-fluoro-nicotinate (18.4 g, 0.09 mol, yield: 25%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com