New application of chromaffin cell of adrenal medulla or opium peptide energy cell
A technology of chromaffin cells and adrenal glands, applied in the field of adrenal medulla chromaffin cells or opioid peptidergic cells, can solve the undiscovered APA microencapsulated bovine adrenal medulla chromaffin cell withdrawal syndrome and other problems, and achieve good immunity Protective effect, less irritating and oppressive side effects, effect of alleviating withdrawal symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Isolation and purification of BCC
[0042] 1. Get 12 fresh bovine adrenal glands from the slaughterhouse (the ischemia time at room temperature is no more than 1 hour), and transport them back to the laboratory quickly under refrigerated conditions for future use.
[0043] 2. Inject collagenase I (collagenase I) solution with a concentration of 1g / L from the vein of the adrenal glands, inject 5ml of collagenase I solution into each adrenal gland, and then place it at 37°C for 30 minutes to allow the collagenase to interact with bovine adrenal cells The colloids are fully responsive.
[0044] 3. Cut the cortex along the longitudinal axis of the adrenal gland, separate the medulla and cut it into pieces.
[0045] 4. Add 60ml of collagenase I solution with a concentration of 1g / L to all the chopped medullary tissue, and let it stand for another 30 minutes.
[0046] 5. Filter with a steel mesh with a mesh of 170 mesh (88 μm) to collect the liquid under the m...
Embodiment 2
[0052] Embodiment 2: Preparation of microencapsulated animal cell drug
[0053] 1. Use the BCC obtained by the method of Example 1, and centrifuge to obtain the precipitate of BCC, wash with physiological saline and dilute to 1 ml, and move it into a centrifuge tube.
[0054] 2. Counting by disc blue staining method, the total number of cells obtained is 3×10 6 cells, in which the purity of BCC was 82%.
[0055] 3. Add 1ml of sodium alginate solution with a concentration of 15g / L to it, and make it into a suspension by stirring.
[0056] 4. Spray the suspension into 100ml of calcium chloride solution with a concentration of 100mmol / L using an electrostatic droplet generator (electrostatic droplet generator, manufactured by the University of Toronto, Canada), and obtain a diameter of 180-500 μm after 10 minutes. Calcium alginate beads containing cells within the range were precipitated, and the supernatant was removed after the precipitation was complete.
[0057] 5. Add cal...
experiment example 1
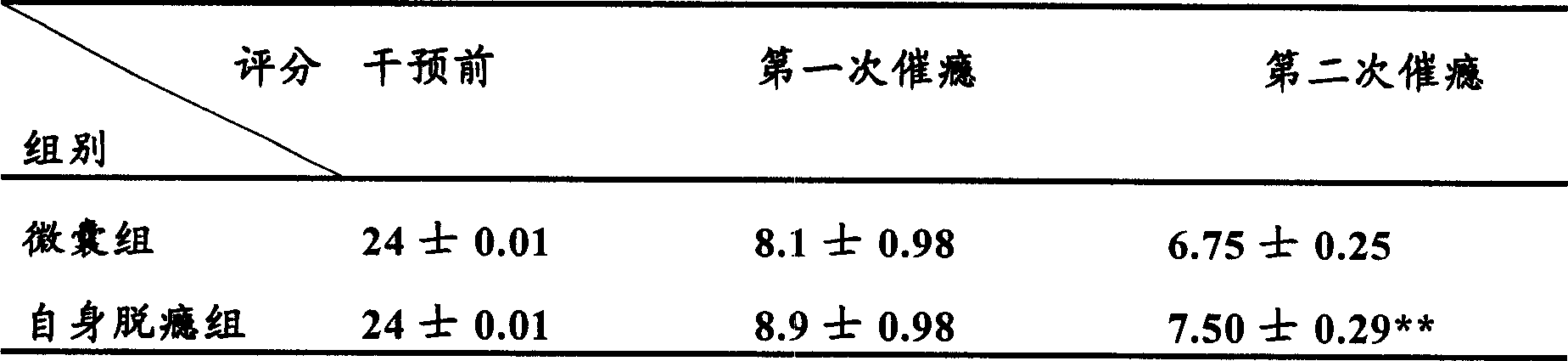
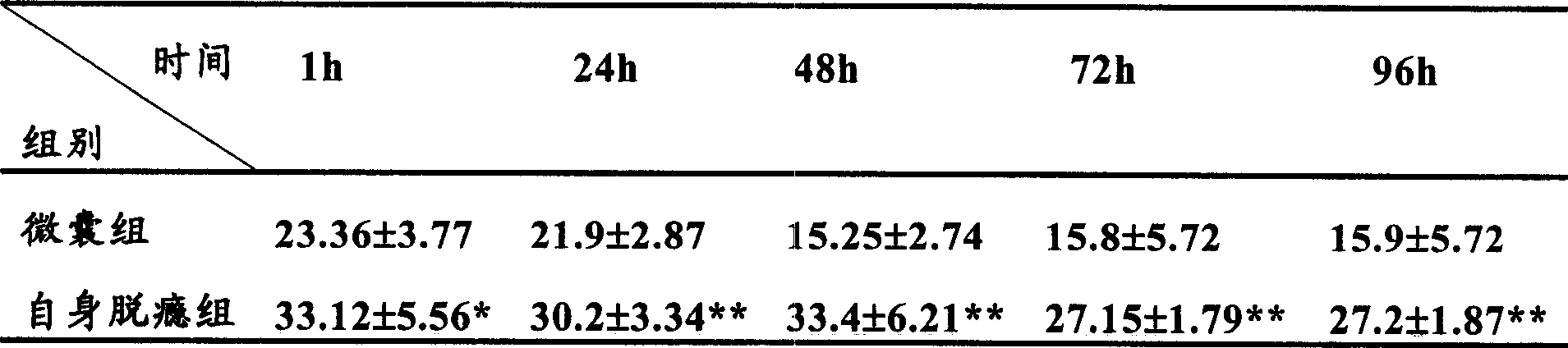
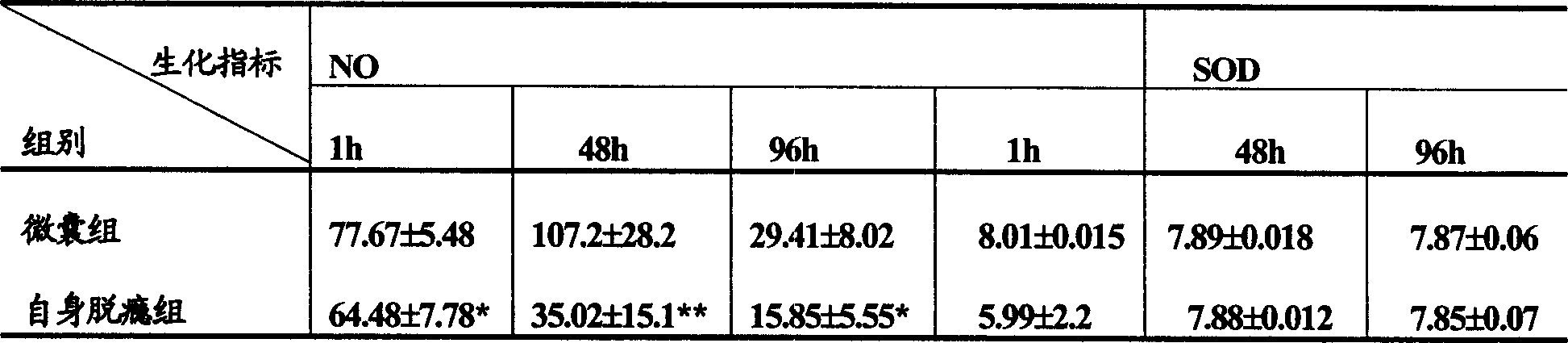
[0061] Experimental example 1: Effect of microencapsulated BCC on withdrawal syndrome in rats
[0062] Experimental Materials
[0063] 1 Experimental drugs: morphine hydrochloride, methadone, naloxone hydrochloride, normal saline, NO kit, SOD kit, prostaglandin E 2 Radioimmunization Kit, Heparin Sodium Injection, Indomethacin Injection, Chloral Hydrate Injection.
[0064] 2 Microencapsulated chromaffin cells.
[0065] 3 Experimental animals: Wistar rats, about 250g.
[0066] experimental method
[0067] 1 Establishment of rat morphine-dependent model (referring to the method of Ling GSF et al.): A total of 150 Wistar rats with a body weight of about 250 g, half male and half male, were randomly divided into 5 groups, with 30 rats in each group. Groups 1-4 are the morphine-dependent model group. Morphine is injected subcutaneously twice a day (8:00 am and 6:00 pm). The dose starts from 20 mg / kg body weight per day and increases by 20 mg / kg body weight per day for 5 days. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com