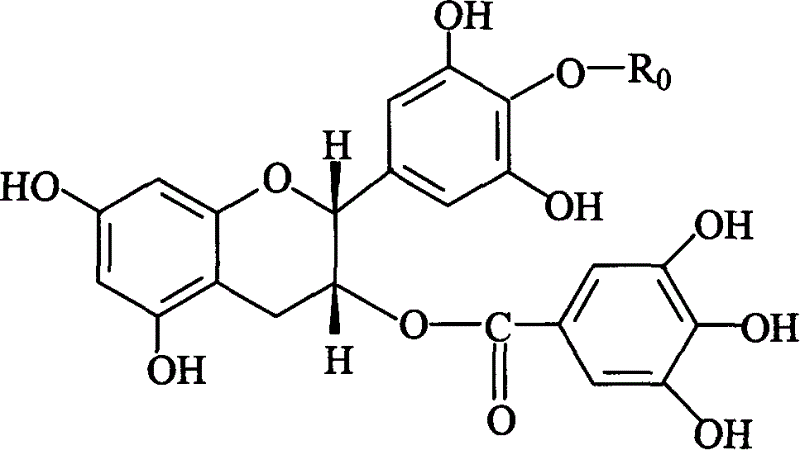
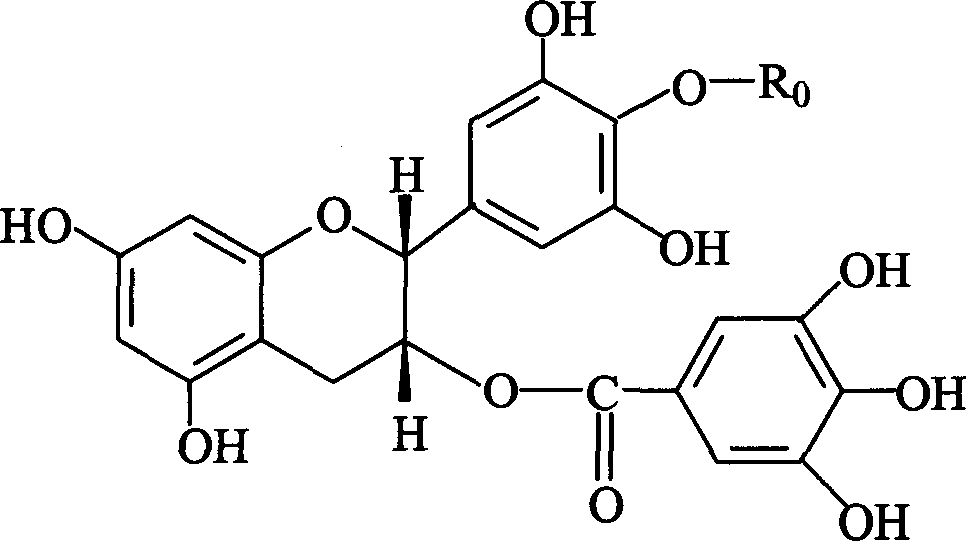
Sensitizing energistic agent of chemotherapy medicine for treating tumour
A technology of chemotherapeutic drugs and synergists, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, and can solve the problems of unsustainable drug effects, easy decomposition, and affecting efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Tumor inhibitory effect when fat-soluble EGCG is used in combination with doxorubicin
[0058] Firstly, the nude mouse KB-A-1 cell solid tumor model was established according to the conventional method, and then the tumor inhibition test in Example 1 was carried out in random groups.
[0059] Specific conditions for the antitumor test using fat-soluble EGCG in combination with the antitumor drug doxorubicin:
[0060] Group 1 is a blank group that only gives normal saline and does not administer drugs;
[0061] The second group is the administration of 5 μg / ml doxorubicin drug group;
[0062] The third group was the group that administered 40 mg / kg fat-soluble EGCG;
[0063] Group 4 was administered with 20 mg / kg fat-soluble EGCG+5 μg / ml doxorubicin;
[0064] Group 5 was administered with 40 mg / kg fat-soluble EGCG+5 μg / ml doxorubicin.
[0065] The experimental results are shown in the table below:
[0066] group
[0067] From the comparison of th...
Embodiment 2
[0068] Example 2 The antitumor effect of fat-soluble EGCG combined with antitumor drug vincristine.
[0069] Firstly, nude mouse KB-A-1 cell solid tumor models were established according to conventional methods, and then the tumor inhibition experiments were performed in random groups.
[0070] Specific conditions for the anti-tumor test using fat-soluble EGCG and vincristine in combination:
[0071] The first group was given normal saline, and the blank group without drugs;
[0072] The 2nd group is to apply the vincristine drug group of 4 μ g / ml;
[0073] The third group was the group that administered 40 mg / kg fat-soluble EGCG;
[0074] The fourth group was the drug group of 20mg / kg fat-soluble EGCG+4μg / ml vincristine;
[0075] Group 5 was given 40mg / kg fat-soluble EGCG+4μg / ml vincristine drug group.
[0076] The experimental results are listed in the table below.
[0077] group
1
2
3
4
5
Average tumor weight (g)
1.61...
Embodiment 3
[0080] Example 3 Antitumor effect of fat-soluble EGCG combined with antitumor drug mitomycin.
[0081] Firstly, nude mouse KB-A-1 cell solid tumor models were established according to conventional methods, and then the tumor inhibition experiments were performed in random groups.
[0082] The specific conditions for the anti-tumor test using fat-soluble EGCG in combination with the anti-tumor drug mitomycin:
[0083] Group 1 is a blank group that only gives normal saline and does not administer drugs;
[0084] Group 2 was the drug group administered with 4 μg / ml mitomycin;
[0085] The third group was the group that administered 40 mg / kg fat-soluble EGCG;
[0086] The 4th group is the administration group of 20mg / kg fat-soluble EGCG+4μg / ml mitomycin;
[0087] Group 5 was administered with 40mg / kg fat-soluble EGCG+4μg / ml mitomycin.
[0088] The experimental results are listed as follows:
[0089] group
1
2
3
4
5
Average tumor w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com