Granisetron hydrochloride orally disintegrating tablets
A technology of granisetron hydrochloride and orally disintegrating tablets, which is applied in the direction of pill delivery, digestive system, drug combination, etc., can solve the problems of incomplete dispersion, disintegration of orally disintegrating tablets, poor tablet hardness and integrity, and achieve High dissolution rate, overcoming disintegration, fast disintegration and dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
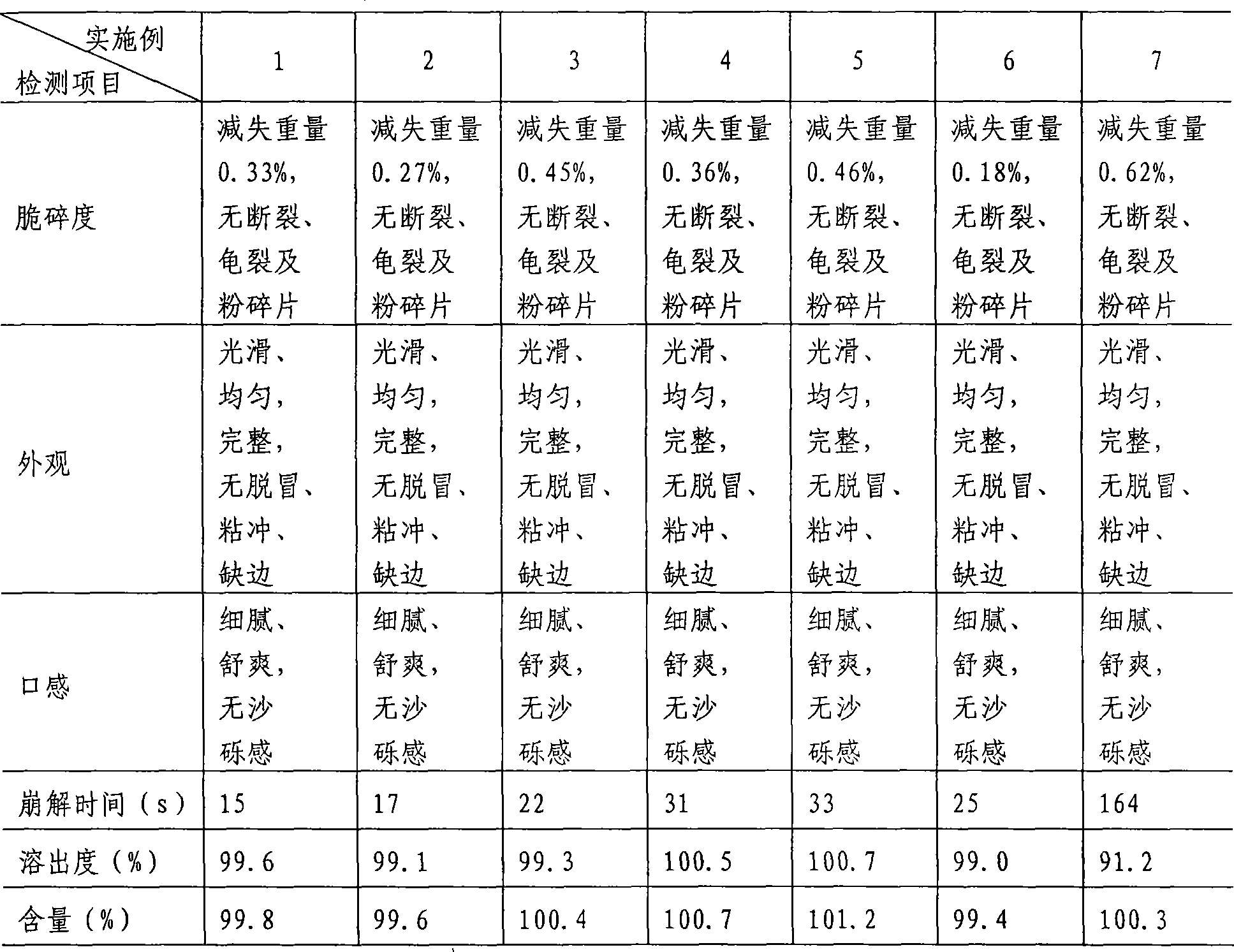
Examples
Embodiment 1
[0052] Embodiment 1 Melting hot extrusion method
[0053] Prescription: Granisetron Hydrochloride 1g
[0054] Macrogol 6000 5g
[0055] Mannitol 40g
[0056] Microcrystalline Cellulose 25g
[0057] Low-substituted hydroxypropyl cellulose 5g
[0058] Croscarmellose Sodium 5g
[0059] Cross-linked polyvinylpyrrolidone 5g
[0060] Citrate 0.5g
[0061] Stevia 1g
[0062] Flavor 1g
[0063] Micronized silica gel 0.5g
[0064] Magnesium stearate 0.5g
[0065]
[0066] Makes 1000 pieces
[0067] Preparation method: Take the prescribed amount of granisetron hydrochloride and add it to the prescribed amount of polyethylene glycol 6000 heated at 90-100°C to melt, stir well and evenly to form a molten mixture, and take it out under negative pressure with a melting heat extruder. Melt the mixed material, cool it down to 40°C under gradient cooling, extrude it with compressed air, granulate it through a sieve plate with a rotary swinging...
Embodiment 2
[0068] Embodiment 2 melt hot extrusion method
[0069] Prescription: Granisetron Hydrochloride 1g
[0070] Macrogol 6000 10g
[0071] Mannitol 20g
[0072] Microcrystalline Cellulose 20g
[0073] Low-substituted hydroxypropyl cellulose 10g
[0074] Croscarmellose Sodium 10g
[0075] Cross-linked polyvinylpyrrolidone 10g
[0076] Citrate 0.5g
[0077] Stevia 1g
[0078] Flavor 1g
[0079] Micronized silica gel 1g
[0080] Magnesium stearate 0.5g
[0081]
[0082] Makes 1000 pieces
[0083] Preparation method: Take the prescribed amount of granisetron hydrochloride and add it to the prescribed amount of polyethylene glycol 6000 heated at 90-100°C to melt, stir well and evenly to form a molten mixture, and take it out under negative pressure with a melting heat extruder. Melt the mixed material, cool it down to 45°C under gradient cooling, extrude it with compressed air, granulate it through a sieve plate with a rotary swinging...
Embodiment 3
[0084] Embodiment 3 melting hot extrusion method
[0085] Prescription: Granisetron Hydrochloride 1g
[0086] Macrogol 6000 10g
[0087] Mannitol 30g
[0088] Microcrystalline Cellulose 40g
[0089] Low-substituted hydroxypropyl cellulose 5g
[0090] Croscarmellose Sodium 10g
[0091] Cross-linked polyvinylpyrrolidone 5g
[0092] Citrate 2g
[0093] Stevia 1g
[0094] Flavor 1g
[0095] Micronized silica gel 2g
[0096] Magnesium stearate 0.5g
[0097]
[0098] Makes 1000 pieces
[0099] Preparation method: Take the prescribed amount of granisetron hydrochloride and add it to the prescribed amount of polyethylene glycol 6000 heated at 90-100°C to melt, stir well and evenly to form a molten mixture, and take it out under negative pressure with a melting heat extruder. Melt the mixed material, cool it down to 50°C under gradient cooling, extrude it with compressed air, granulate it through a sieve plate with a rotary swinging ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com