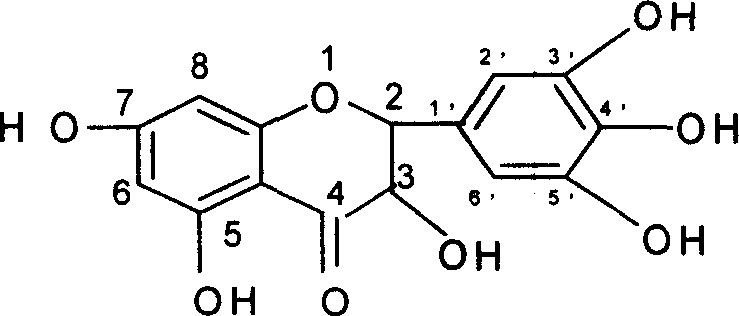
Dihydromyricitrin fatty ester preparing process
A technology of dihydromyricetin and fatty acid esters, which is applied in the direction of organic chemistry and can solve the problems of dihydromyricetin insoluble in fat and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Take 2.00g of dihydromyricetin, dissolve (dilute) with 20ml of ethyl acetate, add 5g of potassium bicarbonate, heat to 40°C, add 1.2-10g of palmitoyl chloride dropwise within 30min, continue to keep warm for 1.5h and then filter, the filtrate Washed with water, concentrated under reduced pressure, recrystallized, and then dehydrated and dried to obtain dihydromyricetin palmitate.
[0014] Example 1
[0015] Take 10.00g of dihydromyricetin, dissolve (dilute) with 100ml of ethyl acetate, add 20g of potassium bicarbonate, heat to 80°C, add 10-20g of stearic acid dropwise within 60min, continue to keep warm for 3h and then filter, the filtrate is passed through Wash with water, concentrate under reduced pressure, recrystallize, and then dehydrate and dry to obtain dihydromyricetin stearate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com