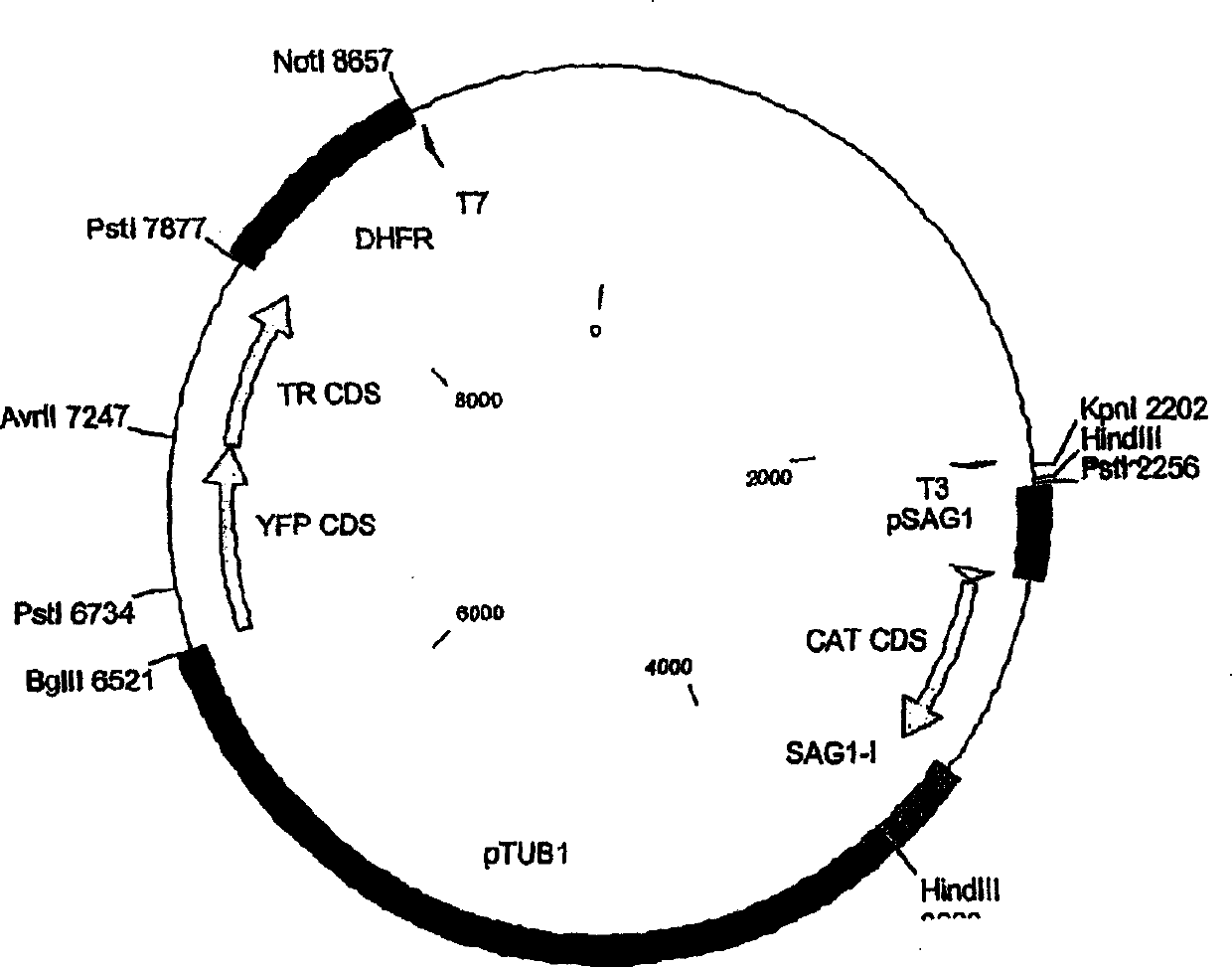
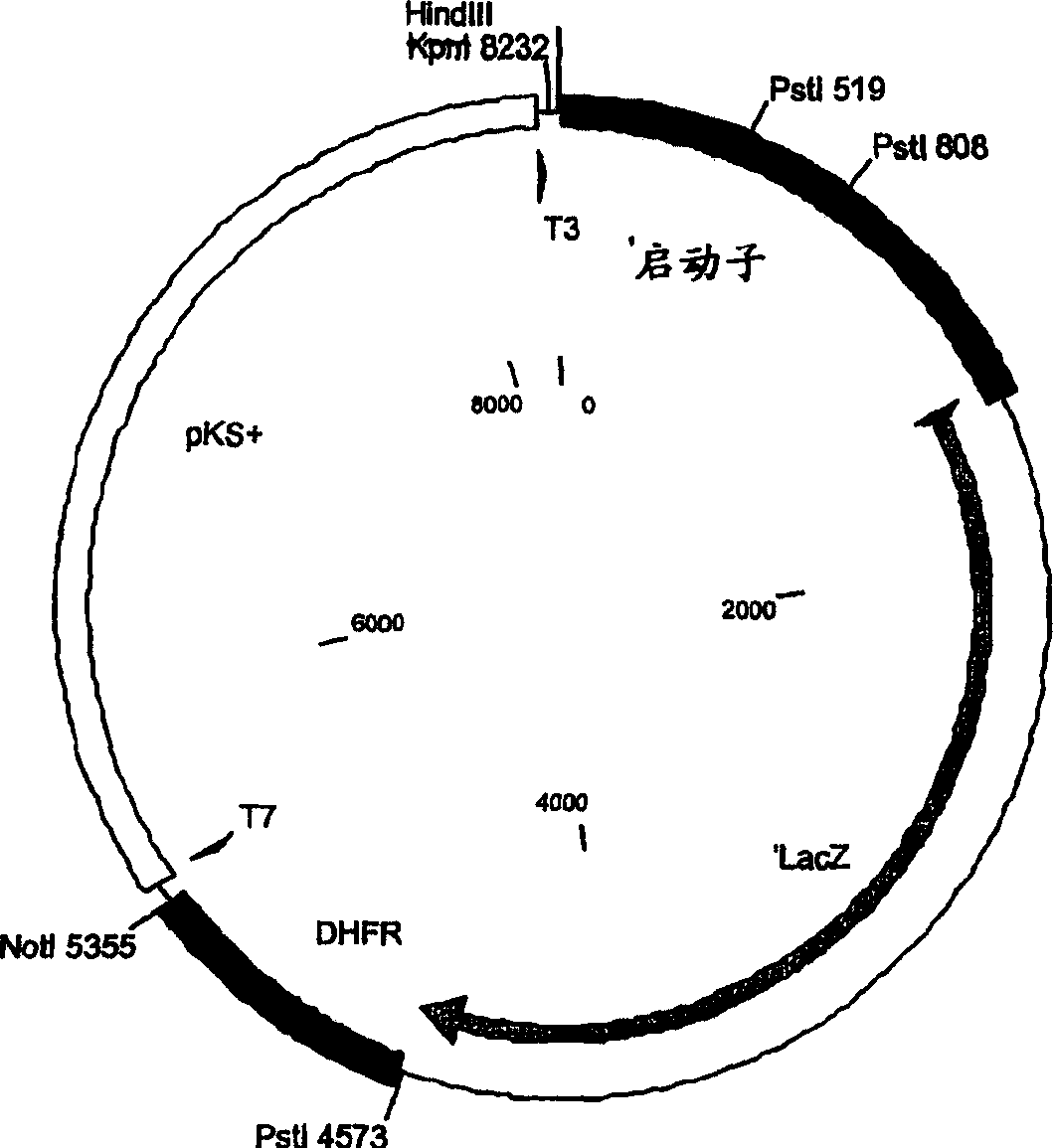
Live antenuated parasite vaccine
A technology for parasites and vaccines, applied in the direction of complete cells/viruses/DNA/RNA components, animal/human peptides, antibody mimics/scaffolds, etc., can solve the problems of not providing local immunity, expensive, not very successful, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Primers used during the experimental procedure:
[0155] Inserted restriction sites are underlined.
[0156] SEQ ID NO # Name Sequence 5'→3'
[0157] 5 1 SAG3-FW CGAT AAGCTT CGAATCTCTGAACGGATGTGT
[0158] 6 2 TUB5-RV CG AGATC TGGGAATTCAAGAAAAAATGCCAACG
[0159] 7 3 TETAVR5-FW CGAT CCTAGG ATGTCTAGATTAGATAAAAG
[0160] 8 4 TETPST3-RV CGT CTGCAG TTAAGACCCACTTTTCACATTTAAG
[0161] 9 5 T3 ATTAACCCTCACTAAAGGGAA
[0162] 10 6 SAG1 / 1634-RV CGAT AAGCTT TCGGGGGGGCAAGAATTGTGT
[0163] 11 7 REV 13A GCGCCCCATGGTGACGGAGAAAAATCG
[0164] REV 13B (Nested
[0165] 12 8 GGGAACCGCAAGGTGGGAGCGGAGAAC
[0166] Primer)
[0167] 13 9 S13PROMFUS FW GCAT AAGCTT CCTCGCAGAGATTGTCAGTG
[0168] 14 10 S13PROMFUS RV GCATT CTAGA GGCAGACATGCCCTTTCCAGG
[0169] 15 11 LACZ-AVRII FW CGAT CCTAGG ATGACCATGATTACGGATTCACT
[0170] 16 12 LACZ-PSTI RV CGAT CTGCAG TTATTTTTGACACCAGACCAA
[0171] GGTTTCCCCCTCAAATCCCTAT...
Embodiment 2
[0200] Determination of the Initiation Transcription Site of S13 Ribosomal Protein Gene of Toxoplasma gondii
[0201] To determine the initiation of transcription of the ribosomal protein gene S13, RNA was isolated from murine Toxoplasma gondii RHΔHXGPRT tachyzoites grown in Vero cells. Gene-specific full-length cDNA was obtained from total RNA using the GeneRacer(R) kit (Invitrogen). Using this kit, oligo RNA is ligated to the end of full-length mRNA, and after reverse transcription with oligo dT, the product is amplified by PCR using GeneRacer primers combined with oligo RNA and gene-specific primers. The start of transcription (STS) can then be determined. For ribosomal protein gene S13, this can be done using the following primers: REV13A (#7, SEQ ID NO: 11) and REV13B (#8, SEQ ID NO: 12). Primer #7 was used with the GeneRacer primer to obtain the product, followed by primer #8 for nested PCR. The PCR product showed 3 bands; 2 weak and 1 strong. The band showing the gr...
Embodiment 3
[0203] S13 / LZ construct
[0204] To test the induced expression of the tet repressor, several reporter constructs were made using the lacZ gene under the control of the S13 promoter with and without the presence of a single tetO site. First, plasmid S13 / lacZ was prepared (see Figure 2 for structure and sequence of the final construct), which was then used to insert or replace the tetO site sequence as described below.
[0205] The S13 primer was amplified by PCR from genomic DNA of the murine Toxoplasma gondii RH / ΔHXGPRT strain using primers S13PROMFUS FW (#9, SEQ ID NO: 13) and S13PROMFUS RV (#10, SEQ ID NO: 14) sub-area. The lacZ coding sequence was amplified by PCR from genomic DNA of BL21 bacteria using primers LACZ-AVRII FW (#11, SEQ ID NO: 15) and LACZ-PSTI RV (#12, SEQ ID NO: 16). Subsequently, the S13 PCR product was digested with HindIII and XbaI, while the lacZ PCR product was digested with AvrII and PstI. The plasmid ptubYFP / YFP-sagCAT was used to exchange the pt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com