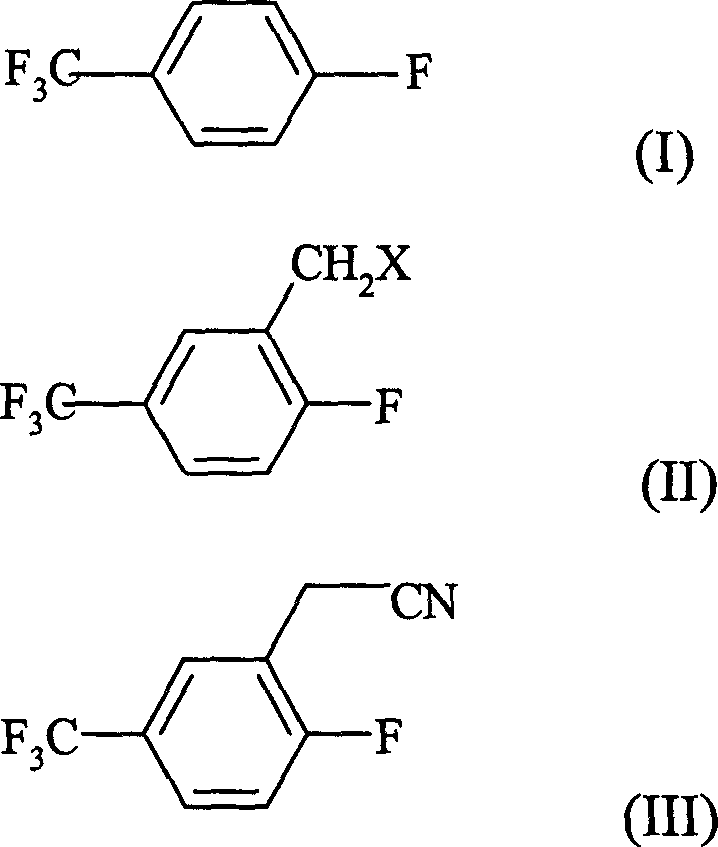
Prepn process of 2-fluoro-5-trifluoromethyl benzyl cyanide
A technology of trifluoromethyl benzene acetonitrile and fluorotrifluorotoluene is applied in the field of preparation of 2-fluoro-5-trifluoromethyl benzene acetonitrile, can solve the problems of high cost, complicated preparation method and the like, achieves high yield, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0015] (1) Preparation of 2-fluoro-5-trifluoromethylbenzyl chloride
[0016] Put 229g of concentrated sulfuric acid into a 250ml four-neck flask, add 24.2g of paraformaldehyde in batches under ice water cooling, control the reaction temperature not to exceed 30°C, and continue stirring for 0.5h after addition. Add 43.5 g of thionyl chloride dropwise within 4 hours at 20-25°C, and continue stirring for 1 hour after the dropwise addition is complete. Add 20.1 g of p-fluorobenzotrifluoride to the reaction flask dropwise within 2 hours at 25-30°C, and react for 4 hours at the same temperature after addition. After the reaction was completed, the mixture was allowed to stand for stratification, and 23.8 g of the upper organic phase was separated.
[0017] Recycle the concentrated sulfuric acid mother liquor after the above reaction, add 4.8g of paraformaldehyde, 9.7g of thionyl chloride, and 20.1g of p-trifluorotoluene, and the other reaction conditions are the same as above to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com